More Information
Submitted: 11 November 2019 | Approved: 20 November 2019 | Published: 21 November 2019
How to cite this article: Seban A, Blein E, Perez S, Seban B. Cranio-Facial Fibrous Dysplasia: A case report of a conservative treatment in a monostotic form associated with an orthodontic management and a bone graft of the non-lytic bone area for dental implant rehabilitation. J Clin Adv Dent. 2019; 3: 018-022.
DOI: 10.29328/journal.jcad.1001011
Copyright License: © 2019 Seban A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Fibrous dysplasia; Monostotic cranio-facial dysplasia; Bone defect; Bone graft; Dental implant; Oral surgery
Cranio-Facial Fibrous Dysplasia: A case report of a conservative treatment in a monostotic form associated with an orthodontic management and a bone graft of the non-lytic bone area for dental implant rehabilitation
Seban A1*, Blein E2, Perez S2 and Seban B2
1Head of Department of Oral Surgery, Fondation A de Rotschild, Paris, France
2Oral Surgeon, Faculty of Dentistry, University of Paris Diderot, Paris, France
*Address for Correspondence: Alfred Seban, Head of Department of Oral Surgery, Fondation A de Rotschild, Paris, France, Email: [email protected]
Fibrous dysplasia is an osteolytic lesion in which bone is replaced by an instable fibrous osseous tissue. The aim of this case report is to highlight dental rehabilitation (bone grafts to allow dental implant) on patients suffering of this condition.
A 39-year-old female with a hard-traumatic event in childhood desired a dental implant rehabilitation on her teeth 19 and 30 after an orthodontic alignment. A Cone-Beam Computed Tomography (CBCT) was performed showing a massive radiopaque lesion of the anterior mandible. The bone grafts and dental implants were successfully managed. A non-invasive treatment with regular follow up was chosen for this case. No evolution was noticed twenty-four month later at the follow up CBCT.
Fibrous dysplasia is a relatively rare non-malignant osteolytic lesion in which bone is replaced by a structurally instable fibro-osseous tissue. Most of the time it is a serendipitously diagnosis as a result of radiography control on children and young adults. This developmental disorder of bone can affect one bone (monostotic form), multiple bone (polyostotic form) or may occur in combination with hyperfunctioning endocrinopathies and hyperpigmented skin lesions as “café-au lait skin macules” (in the setting of Mc Cune-Albright syndrome: MAS). Fibrous Dysplasia is also found in Mazabraud’s syndrome, an atypical benign disease where soft tissue myxoma is associated with MAS.
This slowly progressive tumor, on a rare case, may turn into a malignant tumor [1,2] therefore several authors recommend that patients with Fibrous Dysplasia and a history of surgery should be followed up, for the osteolytic lesions in the operative areas strongly indicate the malignant transformation.
The precise prevalence of the various forms of Fibrous Dysplasia are unknown but this bone disease has been reported to account for approximatively 5% of benign bone lesions; monostotic form is more common and usually occurs in adolescents and young adults. The polyostotic form [3] and the association of Fibrous Dysplasia with extra skeletal manifestation is possible but uncommon.
The history of fibrous dysplasia reveals lesions enlarge in proportion to skeletal growth in monostotic forms while in polyostotic forms severe deformities appear and continue to enlarge after skeletal maturity with development of pathologic fractures [4].
Etiology
Fibrous dysplasia is probably caused by a defective gene, on chromosome 20, that encodes the subunit of a stimulatory G protein (Gsα) processing in bone growth. This disease seems congenital without family or hereditary character despite published cases involving sibling [5,6]. Consequently, the osteoblastic cells will elaborate a fibrous tissue in the bone marrow instead of normal bone [7,8]. A traumatic cause is also evoked: it would be an after-effect of post -traumatic hematomas [9]. Additionally, Calcitonin-resistant osteoclasts activity is possibly involved in the etiology of fibrous dysplasia [10].
Diagnosis
Clinical features: In mild condition low signs and symptoms may appear or none of them. Depending on the anatomic location in cranio-maxillo-facial area, facial asymmetry, deformity, headache, pathological fractures, nerve deficits, orbital swelling, dental complications when the maxillary or the mandible is involved. And in more severe cases diplopia, proptosis, sinus infection, deafness, and loss of vision, increasing swelling, unusual severity of pains are some of the clinical features [11-14]. Thus, representing the major clinical manifestations of the disease.
Radiographic features: Radiographically mixed radiolucent-radiopaque lesions are seen in facial bones, frequently concerning the maxillary, mandible, sphenoid, frontal and ethmoid bones. Initial radiographic assessment, using panoramic and cephalometric radiographs, do not provide a complete analysis of the bone disturbance.
The cone beam computer tomography, on the basis on radiodensity, reveals variable forms of the same lesion process as [15,16], ground glass appearance, orange peel, cottonwood form, heterogenous bone density. The cone beam computer tomography is an important tool for early diagnosis of osteolytic lesions of the disease evaluating a possible extension.
A few studies are related on magnetic resonance imaging signs of fibrous dysplasia and has been described as showing low signal intensity on T1- and T2-weighted magnetic resonance images [17]. However Magnetic resonance imaging features aid to the differential diagnosis, in reflecting the variable tissue of the entity, especially when fibrous dysplasia affects orbital or para-orbital area when low to intermediate signal intensity is seen on both T1- and T2-weighted images [18-20]. “Milk cloud appearance” is a characteristic sign of fibrous dysplasia on contrast-enhanced T1-weighted magnetic resonance imaging and may be helpful when magnetic resonance imaging is performed as the first intention imaging modality [21].
Differential diagnosis of fibrous dysplasia
The review of the literature shows the considerable difficulty occasionally encountered with the differential diagnosis of fibrous dysplasia as follow:
- Fibrous dysplasia and chronic osteomyelitis of the mandible be strongly like each other specifically in the clinical manifestations as well as radiologically. When difficulties are faced in discerning between fibrous dysplasia with periodical pain and swelling, and osteomyelitis, more x-ray examinations must be carried out in relation to the data obtained [22].
- In the skull including facial bones the disease can be mimicked, in rare cases, by a hyperostosis associated with a meningioma due to similar neurological complications [23].
- The differential diagnosis includes also nonossifying fibroma, osteofibrous dysplasia, aneurysmal bone cyst, adamantinoma, exostosis, fracture callus, giant cell tumor, chondrosarcoma, low-grade central osteosarcoma. Histology, history and anatomical location may aid for the diagnosis [24].
Treatment
As a monostotic form of cranio-facial fibrous dysplasia is diagnosed and before any treatment a more severe injury like a polyostotic type must be eliminated by additional investigations such as: bone scintigraphy and eventually endocrinological study with the support of a genetic mutational analysis [25,26].
In monostotic form surgery is suggested when an extension of the disease is confirmed and depending of the evaluation by serial computed tomography scans. Anatomical boundaries, encounter when the cranial base is involved by fibrous dysplasia, do not allow radical surgery and incomplete resection is resulted by a re-growing of the lesion [27]. In case of major deformity, a bone correction and reencountering are recommended. It is also urged to repair a fracture that does not heal. Surgery is also indicated in the prevention of fracture [28].
To avoid damaging surgical act, nitrogenous bisphosphonates are gaining ground in offering pain relief and improving skeletal strength in appropriately selected patients with either polyostotic or monostotic fibrous dysplasia. Particularly the use of pamidronate have showed an inhibition of the resorptive activity of osteoclasts improving the results of the treatment [29].
A 39-year-old female with no significant medical history, except a hard-traumatic event in childhood in the anterior mandibular area, visited our department of oral surgery after an orthodontic alignment of mandibular teeth and space maintenance following premature loss of the first left and right molar that needs to be replaced by two dental implants. At this stage no medical information has been transmitted to us by either the orthodontist or the patient. Intra-oral and extra-oral examinations showed no pathological signs.
A Cone-Beam Computed Tomography (CBCT) was indicated for an accurate placement of implants in both edentulous posterior area of the mandible.
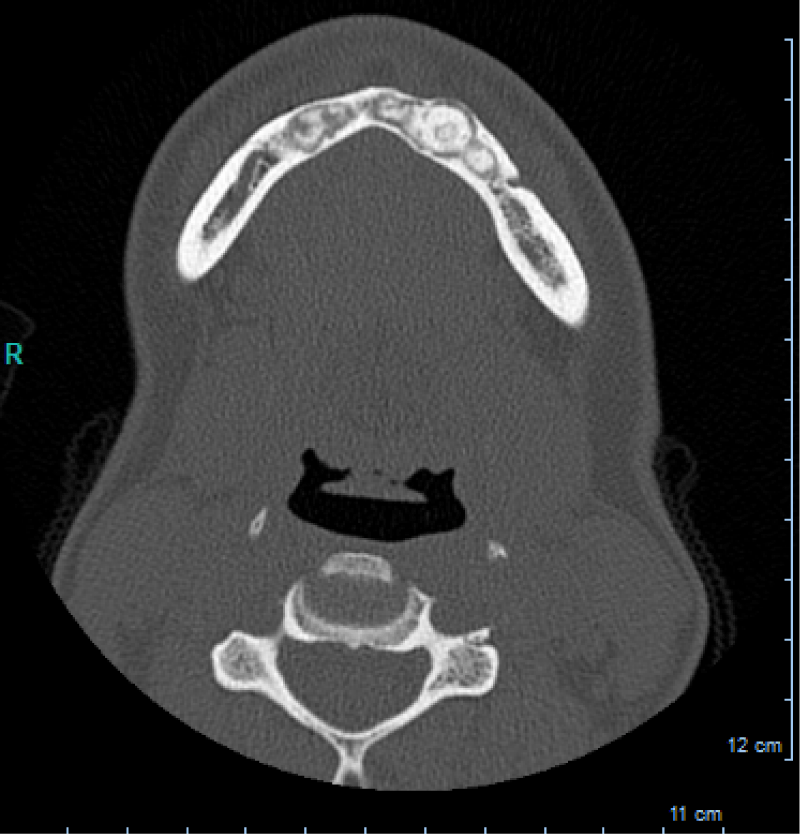
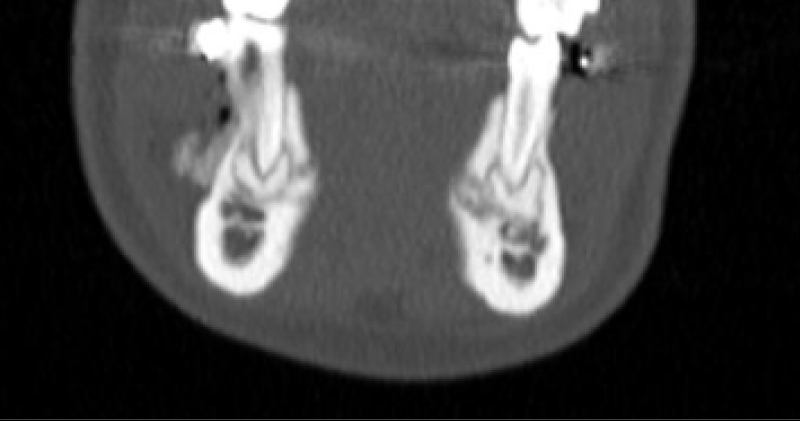
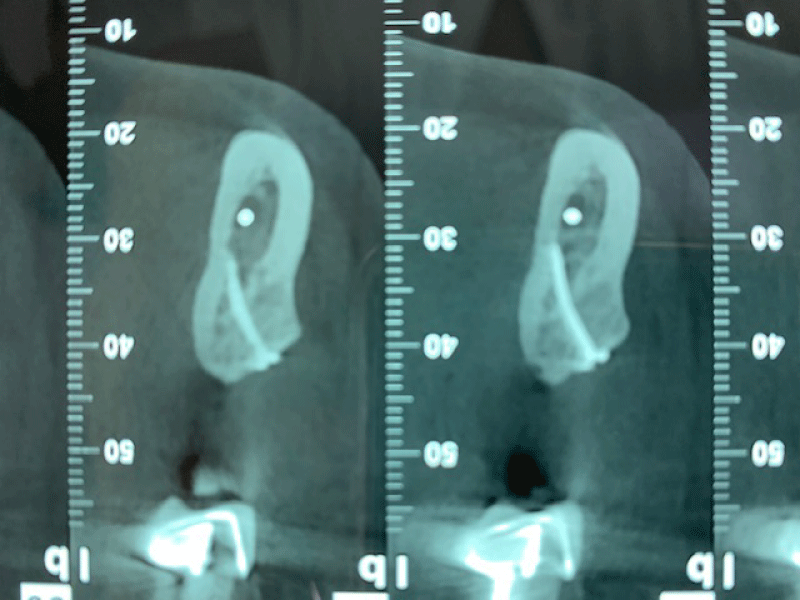
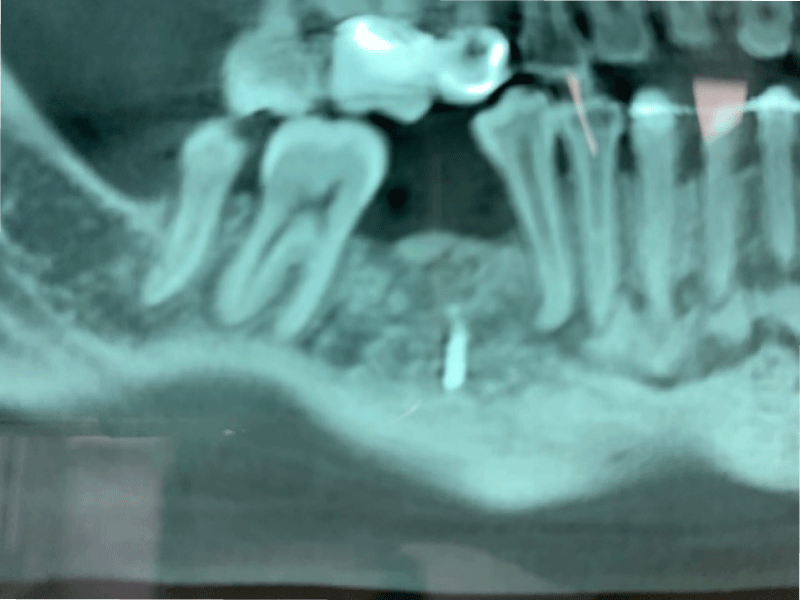
CBCT axial scan shows (Figure 1) a massive radiopaque lesion of the anterior mandible, which a ground glass appearance, confined between the both premolars area without increasing the mandibular body. CBCT coronal sections shows (Figure 2) a lesion with both radiopaque and radiolucent features with well- defined border that did not compromise the buccal and lingual plates (Figure 3). The patient refused the biopsy after becoming aware of the risks associated with this biopsy (dental root lesions, post-operative infection, lesions may be quite vascular and bleeding can be brisk) [30]. CBCT images demonstrate the pathognomonic appearance of fibrous dysplasia. Twenty for month later at follow up CBCT examination, no difference was noticed in the imaging findings. An MRI of the pelvis and the long bones was carried out to rule out a polyostotic fibrous dysplasia.
Figure 1: CBCT axial scan shows a massive radiopaque lesion of the anterior mandible, which a ground glass appearance, confined between the both premolars area without increasing the mandibular body.
Figure 2: CBCT coronal sections shows a lesion with both radiopaque and radiolucent features with well- defined border that did not compromise the buccal and lingual plates.
Figure 3: CBCT coronal sections shows a lesion with both radiopaque and radiolucent features with well- defined border that did not compromise the buccal and lingual plates.
No endocrine abnormalities or pathologies such growth abnormalities were found in this patient in a further comprehensive evaluation.
In conjunction of radiological examinations clinicians recommend, in all cases of fibrous dysplasia, additional testing as blood and hormone testing.
A non- invasive treatment with regular follow up was chosen for this case of monostotic form of cranio- facial fibrous dysplasia limited at the anterior mandibular area without significant extension two years after diagnosis. Lacking of formal scientific evidence it is not possible to relate the trauma suffered in childhood as a possible etiology of fibrous dysplasia: it is an hypothetical cause.
CT scan plays a crucial role in the identification of fibrous dysplasia, determining the lesion, his location and relations to neighboring anatomic structures. Early or late lesions are perfectly identifiable on a CBCT: early-phase lesions appearing radiolucent or mottled and late-phase lesions appearing sclerotic allowing us to assess the stage of the lesion [31,32].
Given that the lesion was stable and in view of the additional medical examinations carried out; a multidisciplinary decision resulted in a bone graft and implant placement without any pathological consequences two years after treatment as shown in the following Figures 4-10:
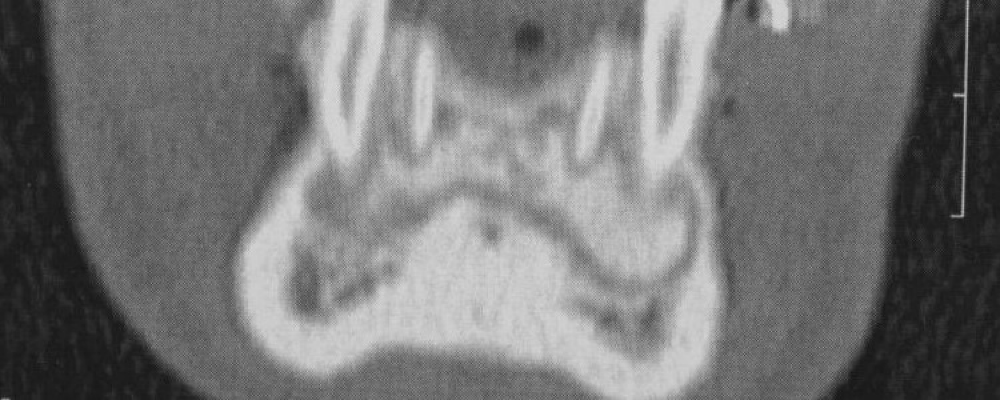
Figure 4: CBCT scan: coronal view of a horizontal bone defect due to the first right molar extraction
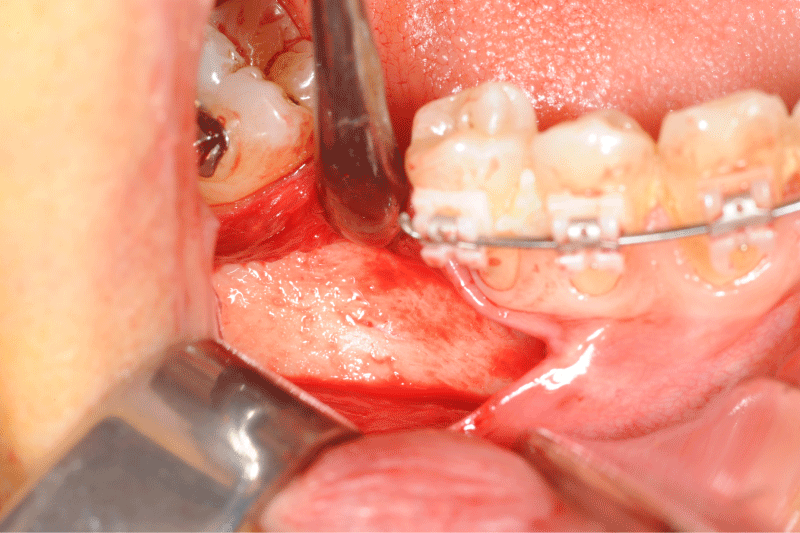
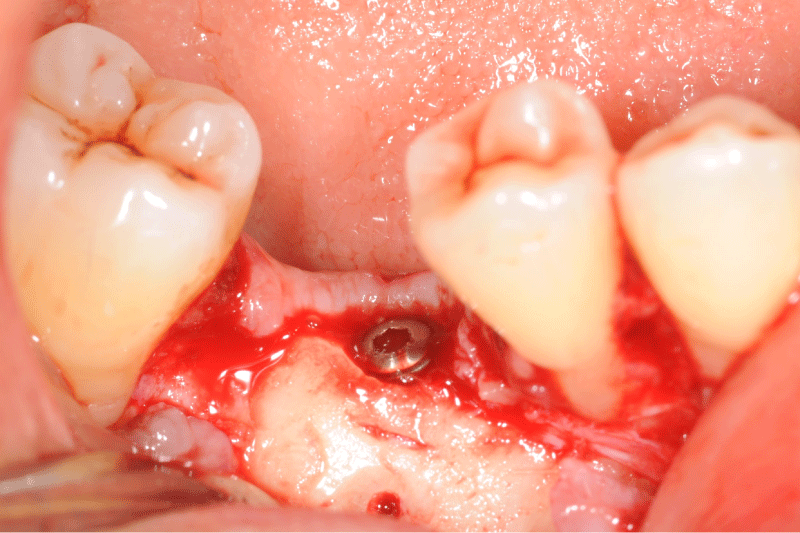
Figure 5: Clinical view of the defect bone after a full thickness flap.
Figure 6: Allogeneic block graft and border void spaces filled with allogeneic particles.
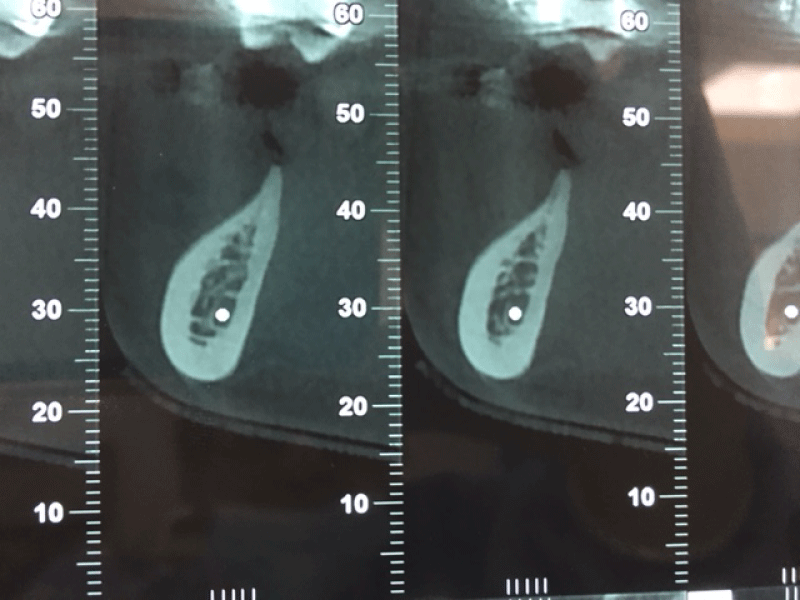
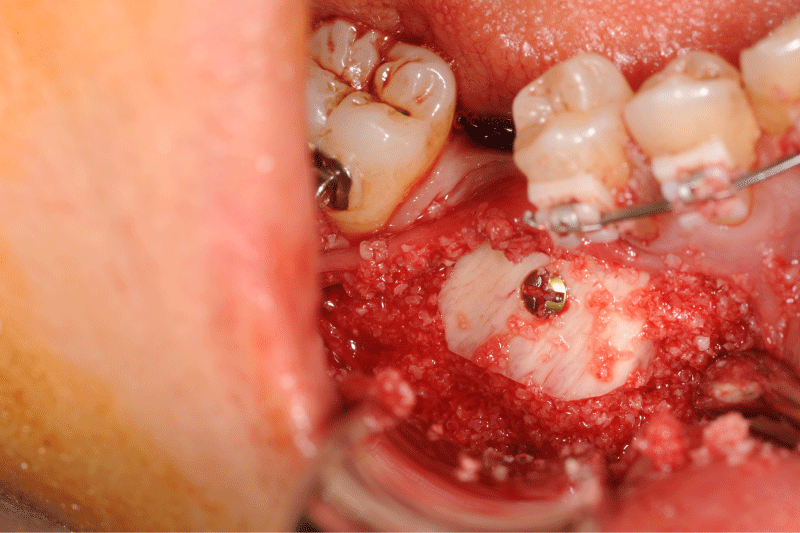
Figure 7: Alveolar bone width assessed at six months using coronal and panoramic CBCT images prior implant placement: the new bone is formed without prior resorption. The block graft was clinically well integrated into the defect site. No other clinical problem was observed.
Figure 8: Alveolar bone width assessed at six months using coronal and panoramic CBCT images prior implant placement: the new bone is formed without prior resorption. The block graft was clinically well integrated into the defect site. No other clinical problem was observed.
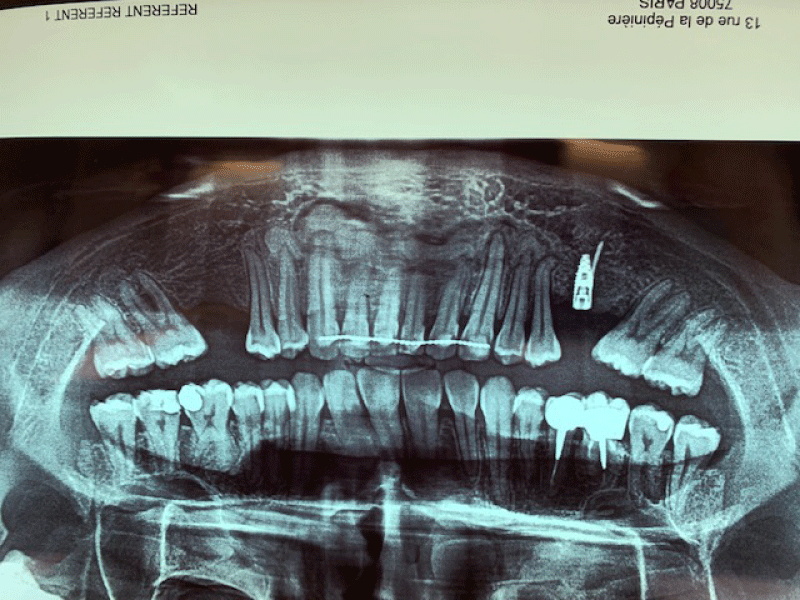
Figure 9: Clinical and panoramic view of implant procedure: The augmented bone remains stable during implant placement the osteosynthesis screw was broken during her removal without any pathological incidence.
Figure 10: Clinical and panoramic view of implant procedure: The augmented bone remains stable during implant placement the osteosynthesis screw was broken during her removal without any pathological incidence.
The clinical behavior and the non-progression in this clinical case of monostotic fibrous dysplasia, thereby making the management less difficult in bone grafting and dental implant placement. We also relied on the fact that monostotic form of fibrous dysplasia may stop growing after skeletal development and only some lesions continue to extend slowly. These last considerations do not prevent a necessary follow-up of the patients to treat them safely, especially when bone grafts and dental implants are involved. This clinical case also allowed us to set the conditions for future research in evaluating patients with mandibular or maxillary monostotic fibrous dysplasia and requiring pre-implant or implant treatments.
- Pack SE, Al Share AA, Quereshy FA, Baur DA. Osteosarcoma of the Mandible Arising in Fibrous Dysplasia-A Case Report. J Oral Maxillofac Surg. 2016; 74: 2229.e1-2229.e4. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27425882
- Qu N, Yao W, Cui X, Zhang H. Malignant transformation in monostotic fibrous dysplasia: clinical features, imaging features, outcomes in 10 patients, and review. Medicine (Baltimore). 2015; 94: e369. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25621678
- Leet AI, Collins MT. Current approach to fibrous dysplasia of bone and McCune-Albright syndrome. J Child Orthop. 2007; 1: 3-17. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19308500
- DiCaprio MR, Enneking WF. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint Surg Am. 2005; 87: 1848-1864. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16085630
- Hsissen MA, Kadiri F, Zamiati S, Jabri L, Rifki S, et al. A case of facial fibrous dysplasia in brothers. Rev Stomatol Chir Maxillofac. 1997; 98: 96-99. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9324737
- Hunter AG, Jarvis J. Osteofibrous dysplasia: two affected male sibs and an unrelated girl with bilateral involvement. Am J Med Genet. 2002; 112: 79-85. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12239726
- Parekh SG, Donthineni-Rao R, Ricchetti E, Lackman RD. Fibrous dysplasia. J Am Acad Orthop Surg. 2004; 12: 305-313. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15469225
- Sargin H, Gozu H, Bircan R, Sargin M, Avsar M, et al. A case of McCune-Albright syndrome associated with Gs alpha mutation in the bone tissue. Endocr J. 2006; 53: 35-44. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16543670
- Pierre Gangloff, Anne Polo, Hevré Moizan, Nicolas Froment, Eric Gerard. Maxillary tumefaction revealing a fibrous dysplasia. A case report. Med Buccale Chir Buccale. 2004; 10: 77-81.
- Long A, Loughlin T, Towers RP, McKenna TJ. Polyostotic fibrous dysplasia with contrasting responses to calcitonin and mithramycin: aetiological and therapeutic implications. Ir J Med Sci. 1988; 157: 229-234. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2971632
- Edgerton MT, Persing JA, Jane JA. The surgical treatment of fibrous dysplasia. With emphasis on recent contributions from cranio-maxillo-facial surgery. Ann Surg. 1985; 202: 459-479. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3901941
- Singh V, Gupta K, Salunke P. Monostotic craniofacial fibrous dysplasia: report of two cases with interesting histology. Autops Case Rep. 2019; 9. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31321219
- Kaynak BA. Conservative treatment of Fibrous Dysplasia. Pak J Med Sci. 2019; 35: 873-876. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31258611
- Majoor BCJ, Traunmueller E, Maurer-Ertl W, Appelman-Dijkstra NM, Fink A, et al. Pain in fibrous dysplasia: relationship with anatomical and clinical features. Acta Orthop. 2019; 90: 401-405. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31035847
- Kochanowski NE, Badry MS, Abdelkarim AZ, Lozanoff S, Syed AZ. Radiographic Diagnosis of Fibrous Dysplasia in Maxilla. Cureus. 2018; 10: e3127. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30345186
- Chennoju SK, Pachigolla R, Govada VM, Alapati S, Balla S. Idiosyncratic Presentation of Cemento-Osseous Dysplasia - An in Depth Analysis Using Cone Beam Computed Tomography. J Clin Diagn Res. 2016; 10: ZD08-10. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27437374
- Norris MA, Kaplan PA, Pathria M, Greenway G. Fibrous dysplasia: magnetic resonance imaging appearance at 1.5 tesla. Clin Imaging. 1990; 14: 211-215. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2224623
- Wilbur AC, Dobben GD, Linder B. Paraorbital tumors and tumor-like conditions: role of CT and MRI. Radiol Clin North Am. 1987; 25: 631-646. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3107049
- Casselman JW, De Jonge I, Neyt L, De Clercq C, D'Hont G. MRI in craniofacial fibrous dysplasia. Neuroradiology. 1993; 35: 234-237. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8459931
- Shah ZK, Peh WC, Koh WL, Shek TW. Magnetic resonance imaging appearances of fibrous dysplasia. Br J Radiol. 2005; 78: 1104-1115. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16352586
- Franz D, Wechselberger J, Rasper M, Specht K, Kehl V, et al. Milk cloud appearance-a characteristic sign of fibrous dysplasia on contrast-enhanced MR imaging. Eur Radiol. 2019; 29: 3424-3430. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31062134
- Johannsen A. Chronic sclerosing osteomyelitis of the mandible. Radiographic differential diagnosis from fibrous dysplasia. Acta Radiol Diagn (Stockh). 1977; 18: 360-368. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/899860
- Leeds N, Seaman WB. Fibrous dysplasia of the skull and its differential diagnosis. A clinical and roentgenographic study of 46 cases. Radiology. 1962; 78: 570-582. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14463598
- Riddle ND, Bui MM. Fibrous dysplasia. Arch Pathol Lab Med. 2013; 137: 134-138. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23276185
- Alves N, de Oliveira RJ, Takehana D, Deana NF. Recurrent Monostotic Fibrous Dysplasia in the Mandible. Case Rep Dent. 2016; 2016: 3920850. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27340572
- Prada EJ, Hassan KH, Brandi ML, Falchetti A. Polyostotic form of fibrous dysplasia in a 13 years old Colombian girl showing clinical and biochemical response to neridronate intravenous therapy. Clin Cases Miner Bone Metab. 2009; 6: 264-265. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22461257
- Park BY, Cheon YW, Kim YO, Pae NS, Lee WJ. Prognosis for craniofacial fibrous dysplasia after incomplete resection: age and serum alkaline phosphatase. Int J Oral Maxillofac Surg. 2010; 39: 221-226. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20083386
- Xiang Fang, Hongyuan Liu, Yun Lang, Yan Xiong, Hong Duan. Fibrous dysplasia of bone: Surgical management options and outcomes of 22 cases. Mol Clin Oncol. 2018; 9: 98-103. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29977545
- Kos M, Luczak K, Godzinski J, Klempous J. Treatment of monostotic fibrous dysplasia with pamidronate. J Craniomaxillofac Surg. 2004; 32: 10-15. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14729043
- Lee JS, FitzGibbon EJ, Chen YR, Kim HJ, Lustig LR, et al. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis. 2012; 7: S2. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22640797
- Kose TE, Dincer Kose O, Erdem MA, Cankaya A, Ozcan Duman I. Monostotic fibrous dysplasia presenting in maxilla: a case report. J Istanb Univ Fac Dent. 2016; 50: 38-42. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28955564
- Nair PP, Bhargava D, Thomas S, Shreenivas K. Immature fibrous dysplasia: a mixed radio-opaque radiolucent lesion. BMJ Case Rep. 2013; 2013. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23291823