More Information
Submitted: July 23, 2022 | Approved: August 01, 2022 | Published: August 02, 2022
How to cite this article: Picco DR, Lopes LM, Steiner-Oliveira C, Nobre dos Santos M. The protective potential of Carbonic Anhydrase VI (CA VI) against tooth decay in children: A systematic review of the literature. J Clin Adv Dent. 2022; 6: 021-027.
DOI: 10.29328/journal.jcad.1001028
Copyright License: © 2022 Picco DR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Carbonic anhydrases; Saliva; Dental plaque; “Children” and “Dental caries
The protective potential of Carbonic Anhydrase VI (CA VI) against tooth decay in children: A systematic review of the literature
Daniele de Cassia Rodrigues Picco1*, Lenita Marangoni Lopes1, Carolina Steiner-Oliveira2 and Marinês Nobre dos Santos3
1PhD, Department of Dentistry for Children, Pediatric Dentistry Area, Piracicaba Dental School, University of Campinas, 13414-903 - Piracicaba, SP, Brazil
2Professor, Doctor, Department of Dentistry for Children, Pediatric Dentistry Area, Piracicaba Dental School, University of Campinas, 13414-903 - Piracicaba, SP, Brazil
3Free Teacher, Department of Dentistry for Children, Pediatric Dentistry Area, Piracicaba Dental School, University of Campinas, 13414-903 - Piracicaba, SP, Brazil
*Address for Correspondence: Daniele de Cassia Rodrigues Picco, PhD, Department of Dentistry for Children, Pediatric Dentistry Area, Piracicaba Dental School, University of Campinas, 13414-903 - Piracicaba, SP, Brazil, Email: [email protected]
Carbonic anhydrase VI (CA VI) catalyzes the reversible hydration of carbon dioxide in saliva with possible pH regulation, taste perception, and tooth formation effects.
Objective: The aim of this work was to undertake a systematic review regarding the relationship between the expression/activity of CA VI in saliva and in dental biofilm and caries experience.
Study design: Five databases were searched until February 2020. The composition was based on the PRISMA statement and on the PICOS model. First author, year, subject characteristics, analysis performed, outcome, measures & variables were extracted. The used terms were “carbonic anhydrase VI”, “saliva”, “dental biofilm” and “dental caries”.
Results: Five studies in the English language were selected for this systematic review and the main discussed topics were the expression/activity of CA VI in saliva and/or in the dental biofilm of children, and its relationship with dental caries.
Conclusion: Salivary carbonic anhydrase plays an important role in the caries dynamics process since there is an association between the expression/activity of CA VI in saliva and the experience of caries. Thus, this protein can predict the risk of dental caries in young patients.
Carbonic anhydrases (CAs) are zinc enzymes that catalyze the reversible hydration of carbon dioxide in the reaction CO2 + H2O ⇔ HCO3− + H+ and are important for pH homeostasis in body tissues and fluids and for removal of intracellular carbon dioxide [1]. In mammalian cells, CAs are involved in several biological processes, including HCO3− dependent metabolic processes, secretion of electrolytes, respiration, pH regulation, bone resorption, biomineralization, and odontogenesis [2]. Today, 16 different CA isoenzymes have been identified in humans, of which 13 have been shown to be enzymatically actives. Some isoenzymes are expressed in most tissues, while others are tissue- or organ-specific. Five are cytosolic (I, II, III, VII, and XIII), five are membrane-bound (IV, IX, XII, XIV and XV) and two are present in the mitochondria (VA and VB), and one (VI) is a secreted isoenzyme [3].
CA VI is secreted into saliva with considerable individual variation in concentration and activity [4] and it is incorporated in the protein pellicle on tooth enamel and in dental biofilms [5,6]. CA VI supports the neutralization of lactic acid and other acid-producing bacteria through conversion of saliva HCO3− to water and carbon dioxide and pH maintenance through the subsequent buffering phase [7]. CA VI is therefore suggested to be an important enzyme in oral physiology and tooth tissue integrity, i.e., resistance to caries and dental erosion [5,8]. It is possible that besides the concentration, the activity of CA VI in saliva may also be influenced by other variables such as genetic polymorphism found in the coding sequences that may modulate the activity of this isoenzyme [9]. However, data are conflicting as both high and low concentrations and activities of CA VI have been associated with caries [3,10-12].
Typically, the saliva in the mouth has sufficient buffering capacity to neutralize the organic acids produced by bacterial metabolism and repair acid-damaged enamel. However, the increased thickness and density of exopolysaccharide-rich biofilm prevents both diffusions of saliva into the biofilm and acids out of the biofilm [13]. With the progression of this positive feedback loop, the rate of acid damage (demineralization) of the tooth enamel outpaces repair (remineralization), leading to early caries lesion. Considering CA VI in dental biofilm, an early investigation by Kimoto, et al. [5], has demonstrated its activity in dental biofilm and Peres, et al. [9], have claimed that the CA VI presence contributes to the neutralization of plaque acid, mainly in stimulated saliva in which buffering is mainly performed by bicarbonate.
Regarding the role of CA VI in protecting teeth from dental caries, conflicting evidence has been provided in the literature. The study of Szabó [14] has shown that the concentration of CA VI was higher in the saliva of caries-free children. On the other hand, Frasseto, et al. [10] have shown that CA VI activity was higher in the saliva of caries-active children. According to Yan, et al. [15], the total salivary protein, including CA VI, was higher in the caries-active children group. In the same way, the investigation performed by Kivelä, et al. [16] has shown that a low CA VI concentration in saliva was associated with a higher caries index. These latter authors have also found a negative correlation between CA VI concentration and the decayed, missing, and filled teeth index in individuals with poor oral hygiene. In addition, Picco, et al. [11,17] have found that CA VI was shown to be more active in saliva and biofilm of school children with caries. However, Ozturk, et al. [18] have found no significant difference in CA VI concentration when caries and caries-free young adults were compared. In short, the preliminary detection of these isoenzymes may lay some foundation for biomarker research on dental caries susceptibility.
Thus, the recognized clinical importance of this isoenzyme is confronted with the conflicting information presented by the available scientific evidence. Hence, the aim of this paper was to conduct a systematic review of the literature on the protective potential of carbonic anhydrase VI against tooth decay. This review provides a summary of evidence related to the relationship between CA VI expression/activity in saliva and in dental biofilm, by applying explicit methods and systematic search, critical assessment and synthesis of the selected information. The available scientific evidence of CA VI, as well as the relationship between the expression/activity of CA VI in saliva and/or dental biofilm and caries experience, were collected.
Data sources and searches
This systematic review was conducted based on the Preferred Reporting Items for Systematic Reviews available at http://prisma-statement.org [19] and on the PICOS model [20] for the definition of the inclusion criteria as follows: P (Population): “human children from 2 to 12 years old”, I (Intervention: in this case, analysis performed): “Analysis of concentration and/or activity of carbonic anhydrase VI in saliva and/or in dental biofilm”, C (Comparison): “children with no caries experience”; O (Outcomes): “relationship between concentration and/or activity of CA VI in saliva and/or in dental biofilm and dental caries”; and S (Study Design): “transverse or longitudinal study”. The study protocol was not registered. Because this study was a systematic review, ethical approval was not required.
The electronic databases PubMed/MEDLINE, EMBASE, Cochrane Library, PEDRO, as well as the search engine Google Scholar were systematically searched with the search terms (carbonic anhydrase VI) AND (saliva) AND (dental plaque OR biofilm) AND (dental caries) AND (children). The language was restricted to English. All study designs, except systematic reviews, were included. The literature search was concluded on February 1st, 2020.
Data extraction and quality assessment
Two investigators experienced in the signal analysis performed the whole data extraction process independently. Discussion took place after every fulfilled part of the protocol until consensus was achieved. The investigators independently screened all titles for eligibility, based on the prior-defined PICOS inclusion criteria. In case of disagreement, the consensus was achieved through discussion. In the event of controversial persistency, the study was taken to the next step which was the reading of the abstracts. Afterward , the same process was performed to screen the abstracts and finally to screen the full texts for eligibility. If no consensus was found after the full-text reading and discussion, a third investigator decided whether the study should be included or excluded. In the further process, the quality of the included studies and the risk of bias was analyzed using “The Cochrane Collaboration`s tool for assessing the risk of bias” [21]. The criteria list included six items from which each paper was scored with “+” if the criteria were fulfilled, with “-” if the criteria were not fulfilled, or with “?” if the data was not provided or was unclear. According to Viswanathan, et al. [22], the downgrade of the studies not designed as an RCT (randomized controlled trials) was limited by rating the selection bias as unclear risk of bias if the method was appropriate.
Data synthesis and analysis
Data from the selected articles were independently extracted and analyzed by two investigators using a standard-ized extraction form: First author, year; subject characteristics; analysis performed; outcome; measures & variables.
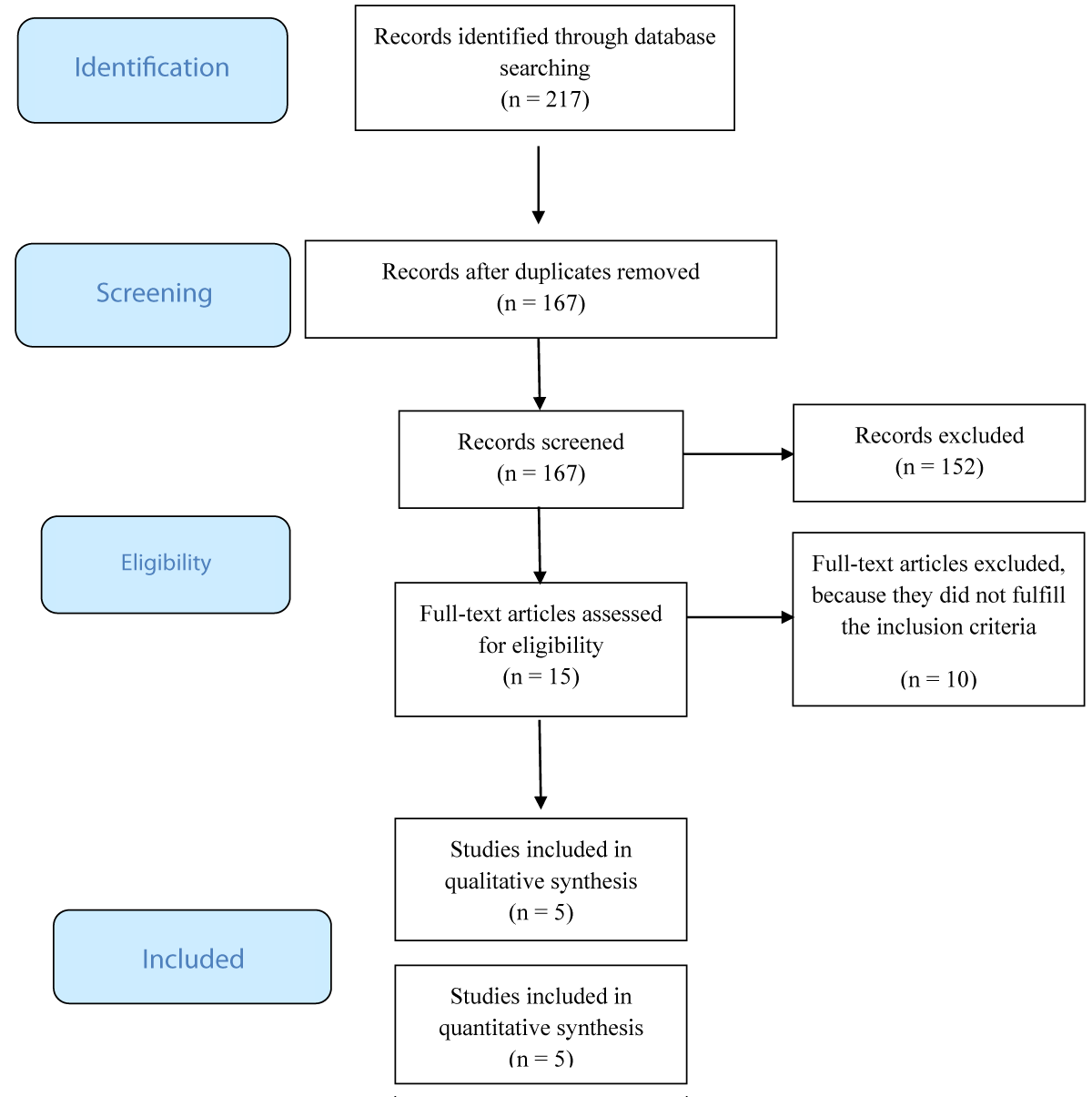
Figure 1 presents the flow chart of the selected studies. The literature search revealed a total of 217 records. After the removal of duplicates, 167 papers remained to be screened on titles and abstracts. A number of papers (n = 152) did not meet the inclusion criteria. Therefore, 15 full-text articles remained to be assessed for eligibility. Another 10 studies had to be excluded because they did not fulfill the inclusion criteria (1 study in adolescents; 4 studies in adults; 1 study in the Chinese language; 4 studies on genetic factors). Finally, 5 studies were included for further analysis.
Figure 1: PRISMA Flow Diagram.
Methodological quality
Following “The Cochrane Collaboration`s tool for assessing the risk of bias”, all studies showed an unclear risk of allocation concealment. All studies showed a low risk of bias concerning the blinding of participants and personnel and 1 study a high risk of selective reporting bias. All studies featured a low risk of other bias, except one due to the unclear description. The summary of bias is depicted in Table 1.
| Table 1: Cochrane risk of bias analysis. | ||||
| Author, year | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Selective reporting (reporting bias) | Other bias |
| Picco, et al. 2019 [17] | ? | + | + | + |
| Picco, et al. 2017 [11] | ? | + | + | + |
| Borghi, et al. 2017 [23] | ? | + | + | + |
| Frasseto, et al. 2012 [10] | ? | + | + | + |
| Szabó, 1974 [14] | ? | + | - | ? |
|
||||
Study characteristics
The activity and concentration of CA VI were investigated in 5 studies. The summary of the data analysis is depicted in Table 2. In the included studies, two main topics were discussed: the expression/activity of CA VI in saliva and in dental biofilm and its relationship with dental caries. These topics are defined in the following section and summarized in Table 2.
| Table 2: Data extraction: Summary of CA VI analysis and dental caries. | ||||
| Main objective | First author, year | Subject characteristics: sample size (n), group-specification, age | Analysis performed | Outcomes, Measures & Variables |
| Correlation between CA VI concentration/ activity and dental caries in dental biofilm. | Picco, et al. 2019 [17] | 74 school children aged 7 - 9 years | pH and the correlation between concentration and activity of CA VI and caries. | CA VI was shown to be more active in the biofilm of school children with caries in order to contribute to the neutralization of biofilm acid. |
| Correlation between CA VI concentration/ activity and dental caries in saliva. | Picco, et al. 2017 [11] | 74 school children aged 7 - 9 years | Salivary flow, pH, buffer capacity and the correlation between concentration and activity of CA VI and caries. | Children with a higher activity of CA VI in saliva and a lower salivary flow rate were more likely to develop dental caries. |
| Relationship between early childhood caries and CA VI activity. | Borghi, et al. 2017 [23] | 37 preschool children aged 2 - 4 years | Correlation among α-amylase, CA VI, and the presence of visible biofilm and early childhood caries. |
No correlation was detected between dental caries and the activity of salivary CA VI. |
| Correlation between CA VI activity and dental caries. | Frasseto, et al. 2012 [10] | 30 preschool children aged 3 - 6 years | The relationship between dental caries and salivary CAVI activity, salivary flow rate, and plaque pH before and after a 20% sucrose rinse. | The pre-rinse CA VI activity and its variation were higher in the saliva from caries children than from caries-free children. |
| Correlation between CA VI concentration and dental caries. | Szabó, 1974 [14] | 52 children aged 7 - 14 years living under heterogeneous and standardized conditions | Investigation about the difference in the CA VI content in the saliva of carious and caries-free groups. | CA VI concentration in saliva of non-carious children was higher than in children with active caries. |
Saliva works as a protective factor of the oral hard and soft tissues as it facilitates clearance of food debris and sugars, contributes to aggregation and elimination of microorganisms, has a buffering capacity to neutralize acids, promotes the remineralization of tooth enamel, and has some antimicrobial properties [23]. Thus, salivary composition and functional properties such as flow rate have been found to be associated with dental caries to varying degrees [24]. Such salivary properties can be used as biomarkers for the risk of future disease and could potentially inform interventions to address this risk [25].
The protein concentration in saliva is approximately 2 mg/ml, about one-thirtieth of that of the plasma so a few amino acids still have a significant buffering effect in the usual pH of the oral cavity. The salivary proteins are also involved in modulating the colonization of microbes on the teeth and soft tissue in the oral mucosa and in the chemical modulation of salivary calcium and phosphate [26]. The salivary proteins also participate in the formation of the acquired enamel pellicle, which is not only protective but also influences the initial microbial colonization on the tooth surface. The production of bases from basic amino acids and peptides in saliva may aid in neutralizing acids in the plaque. Collectively, the salivary proteins, show a broad range of functional activities that can assist in maintaining the integrity of the mouth, as well as in offering protection against oral and non-oral microbial infections [27].
The existence of carbonic anhydrase in human saliva has been known for 60 years [28], but to this day, little is known about the physiological role and the importance of CA VI in the saliva of children. Salivary CA VI, a zinc metalloenzyme is the only known secreted isoenzyme of the CA family, which has been detected in the saliva secreted by the serous acinar cells of the mammalian parotid and submandibular glands. It catalyzes the reaction that bicarbonate ions to neutralize the acids formed by plaque bacteria (CO2 + H2O ↔ HCO3- + H+). By catalyzing this reaction, CA VI is believed to provide a greater buffering capacity to saliva by penetrating dental biofilm and facilitating acid neutralization by salivary bicarbonate [5]. There is evidence suggesting that salivary CA is a multifunctional enzyme, which affects taste bud growth, protection of the teeth from caries, and acts as an anti-inflammatory agent [18].
Salivary CA VI appears to protect teeth from caries via mechanisms other than direct regulation of salivary pH and buffering capacity [29]. In this sense, several researchers have investigated the relationship between the CA VI concentration and caries experience [14-18,30] and between the CA VI activity and caries experience [10,11,23]. The relationship between caries, pH, and buffering effect of the saliva has frequently been examined and some investigators have found that the buffering capacity and pH show a difference when comparing caries-active and caries-free groups [31,32].
As the literature has shown conflicting evidence regarding the relationship between the concentration and activity of CA VI in saliva or in the dental biofilm of subjects with and without caries, this systematic literature review was conducted to critically evaluate this issue. The study designs of the included studies were appropriate regarding their research questions.
The study of Szabó [14] was performed with school children and adolescents aged 7 to 12 years old and has shown that in both stimulated and unstimulated saliva, CA VI concentration was higher in the saliva of caries-free children living under standardized conditions. However, the authors were not able to find a significant difference in CA VI activity in saliva of caries and caries-free subjects when they investigated children who lived and had their meals with their family at home. These results give emphasis the importance of selecting subjects whose nutritional and living conditions are easily controllable, identical, and constant for at least 21/2 years. However, a homogenous sample may not represent the CA VI behavior in the whole population. Moreover, although these authors claimed they determined CA VI activity in saliva, the methodology used in their research made it possible to determine just the concentration of CA VI in saliva. In this view, one should keep in mind that considering the relevance of salivary proteins in the caries dynamic process, it seems more important to investigate the activity of this isoenzyme in saliva and in biofilm. In addition, it is known that during the cariogenic challenges in the oral environment, this active CA VI catalyzes the reversible reaction of carbon dioxide in the reaction of CO2 + H2O ↔ H+ + HCO3- and thus accelerates the neutralization of acid from the local environment of tooth surface [3,5].
In this regard, the investigation performed by Frasseto, et al. [10], has determined the activity of CA VI in the saliva of preschool children and has investigated the relationship between dental caries and salivary CA VI activity, salivary flow rate as well as biofilm pH before and after a 20% sucrose rinse. In this study, the pre-rinse CA VI activity was higher in the saliva of children with dental caries. This result does not agree with those reported by Szabó [14], who found a higher activity of CA VI in caries-free children. However, it should be emphasized that while the methods of analysis employed by the previous authors were able to determine just the expression of salivary CAVI, in the study of Frasseto, et al. [10] the authors used the zymography method [33] to quantitatively determine the activity of CA VI in the saliva of preschool children after a cariogenic challenge. They have also evaluated the variation of CA VI salivary activity after a 20% sucrose rinse and demonstrated that in the saliva of preschool children, the difference between the pre-rinse CA VI activity and the post-rinse CA VI activity was associated with caries. In addition, after a 20% sucrose rinse a decrease in the CA VI activity was observed only in the saliva of children with caries. This finding can be explained by the fact that CA VI catalyzes the reaction of CO2 + H2O ↔ HCO3- + H+ in both directions which means that this isoenzyme may neutralize or acidify the media depending on the oral environment conditions. Besides that, it is known that children who develop caries are more frequently exposed to daily sugar consumption and that this routine is significantly correlated with early childhood caries [34,35]. With regard to salivary flow rate and biofilm pH, no correlation was found between these variables and dental caries.
The longitudinal study of Borghi, et al. [23] was performed with 100 preschool children aging 24–48 months. In this study, the authors have investigated the relationship between CA VI and α -amylase activity in saliva and early childhood caries and have shown that the activity of the CA VI was significantly higher in the saliva of pre-school children with caries than in saliva of caries-free children. Also, the activity of salivary ɑ-amylase was significantly higher in the saliva of caries-free children. The authors have also demonstrated a significant moderate negative correlation between dental caries and the activity of ɑ -amylase. Moreover, no correlation was detected between dental caries and activity of salivary CA VI, despite the authors employing an appropriate number of individuals as evidenced by their sample power calculation. In addition, it was clear that the presence of visible biofilm on the buccal surfaces of upper incisors and low salivary activity of ɑ -amylase increased the risk of children developing early childhood caries. This investigation had some limitations since the authors did not determine the activity of CA VI and α -amylase at the follow-up to add information on how these proteins would behave, as caries remineralize or progresses. In this way, additional longitudinal studies are strongly suggested. In addition, considering that in their study only children with a specific caries pattern and with a narrow age range were investigated, it would be relevant to know how these proteins would behave in saliva and in dental biofilm as children got older.
For this purpose, the study performed by Picco, et al. [11], studied both parameters: isoenzyme concentration and activity and their relationship with dental caries in 74 school children aged 7-9 years old divided into caries and caries-free children. The results have shown that the activity of CA VI isoenzyme in saliva was significantly higher in children with caries than in caries-free children. This study has also demonstrated that the concentration of CA VI was significantly higher in the saliva of caries-free children than in children with caries. In addition, a moderate positive correlation between CA VI activity and concentration was noted in the carious group. Also, a negative weak correlation between saliva pH and CA VI concentration was observed in the caries-free group and a moderate negative correlation between buffering capacity and CA VI activity was observed in the carious group. Their results have finally demonstrated that children with a salivary flow rate lower than 1.06 mL/min have a 3.88 times higher chance of developing caries. In addition, children with an activity of CA VI in saliva higher than 1.75 are 4.88 times more likely to develop caries. These authors suggested that the activity of CA VI in saliva may be considered a biomarker for caries development.
Finally, in the most recent study of this review, Picco, et al. [17] investigated pH, activity, and concentration of CA VI in dental biofilm of caries and caries-free children of 7 - 9 years old divided into caries and caries-free children. The results of this study demonstrated that CA VI is not only concentrated in biofilm, but more importantly, the isoenzyme is active in order to increase acid neutralization after the frequent cariogenic challenges that children with caries are expected to be exposed. Indeed, CA VI activity was significantly higher in the biofilm of children with caries than in the biofilm of caries-free children. This result suggests that the isoenzyme is being more constantly activated in individuals with caries as a protective mechanism to neutralize the pH of the medium, specifically of dental biofilm, to provide a greater defense against enamel demineralization.
As previously discussed, all studies have limitations and, in this sense, future investigations that address the above referred methodological aspects are strongly encouraged. In addition, it is worth noting that this research has as significant limitations the limited number of studies in the literature on the subject addressed, considering the inclusion criteria established for this proposal, besides to the fact that only five studies were included in this systematic review project, which may also have influenced the results obtained. However, the simultaneous information concerning the activity and concentration of CA VI in the saliva is of high clinical relevance because it offers valuable information about its potential protective effect in modulating the caries dynamic process. This knowledge allows us to optimize the experimental protocols for this biomarker, as well as to develop preventive measures against dental caries with increasing precision. These aspects should be considered in future studies.
In conclusion, available data on the scientific evidence about CA VI, as well as the relationship between the expression/activity of CA VI in saliva and in dental biofilm and caries experience suggests that there is an association between the expression/activity of CA VI in saliva and caries experience. However, additional prospective observational cohort studies are strongly encouraged to evaluate if taking into account the relevance of CA VI in saliva and in biofilm, this isoenzyme can be considered a reliable risk factor or risk indicator for caries development.
This paper was based on a thesis submitted by the first author to Piracicaba Dental School, University of Campinas, in partial fulfillment of the requirements for a Ph.D. degree in Dentistry (Pediatric Dentistry area). This work was supported by FAPESP (2012/02886-3 and 2012/15834-1) and FAEPEX (1289/2006) grants.
Authors’ contribution
M. Nobre dos Santos contributed to the conception, design, interpretation of data, drafted the work, and substantively revised this paper; D.C.R. Picco contributed to the conception, design, acquisition, analysis, and interpretation of data, drafted the work and substantively revised this paper; L.M. Lopes contributed to the acquisition, analysis and interpretation of data, and substantively revised this paper. C. Steiner-Oliveira contributed to the content and language revision of this paper. All authors gave their final approval for all aspects of this work.
- Supuran CT. Carbonic anhydrases--an overview. Curr Pharm Des. 2008;14(7):603-14. doi: 10.2174/138161208783877884. PMID: 18336305.
- Reibring CG, El Shahawy M, Hallberg K, Kannius-Janson M, Nilsson J, Parkkila S, Sly WS, Waheed A, Linde A, Gritli-Linde A. Expression patterns and subcellular localization of carbonic anhydrases are developmentally regulated during tooth formation. PLoS One. 2014 May 1;9(5):e96007. doi: 10.1371/journal.pone.0096007. PMID: 24789143; PMCID: PMC4006843.
- Kivela J, Parkkila S, Parkkila AK, Leinonen J, Rajaniemi H. Salivary carbonic anhydrase isoenzyme VI. J Physiol. 1999 Oct 15;520 Pt 2(Pt 2):315-20. doi: 10.1111/j.1469-7793.1999.t01-1-00315.x. PMID: 10523402; PMCID: PMC2269599.
- Kivelä J, Parkkila S, Waheed A, Parkkila AK, Sly WS, Rajaniemi H. Secretory carbonic anhydrase isoenzyme (CA VI) in human serum. Clin Chem. 1997 Dec;43(12):2318-22. PMID: 9439449.
- Kimoto M, Kishino M, Yura Y, Ogawa Y. A role of salivary carbonic anhydrase VI in dental plaque. Arch Oral Biol. 2006 Feb;51(2):117-22. doi: 10.1016/j.archoralbio.2005.04.007. Epub 2005 Jun 14. PMID: 15961059.
- Vitorino R, Lobo MJ, Duarte J, Ferrer-Correia AJ, Tomer KB, Dubin JR, Domingues PM, Amado FM. In vitro hydroxyapatite adsorbed salivary proteins. Biochem Biophys Res Commun. 2004 Jul 23;320(2):342-6. doi: 10.1016/j.bbrc.2004.05.169. PMID: 15219832.
- Bardow A, Moe D, Nyvad B, Nauntofte B. The buffer capacity and buffer systems of human whole saliva measured without loss of CO2. Arch Oral Biol. 2000 Jan;45(1):1-12. doi: 10.1016/s0003-9969(99)00119-3. PMID: 10669087.
- Lips A, Antunes LS, Antunes LA, Pintor AVB, Santos DABD, Bachinski R, Küchler EC, Alves GG. Salivary protein polymorphisms and risk of dental caries: a systematic review. Braz Oral Res. 2017 Jun 5;31:e41. doi: 10.1590/1807-3107BOR-2017.vol31.0041. PMID: 28591238.
- Peres RC, Camargo G, Mofatto LS, Cortellazzi KL, Santos MC, Nobre-dos-Santos M, Bergamaschi CC, Line SR. Association of polymorphisms in the carbonic anhydrase 6 gene with salivary buffer capacity, dental plaque pH, and caries index in children aged 7-9 years. Pharmacogenomics J. 2010 Apr;10(2):114-9. doi: 10.1038/tpj.2009.37. Epub 2009 Sep 1. Erratum in: Pharmacogenomics J. 2010 Jun;10(3):243. Santos, M N [corrected to Nobre-dos-Santos, M]. PMID: 19721466.
- Frasseto F, Parisotto TM, Peres RC, Marques MR, Line SR, Nobre Dos Santos M. Relationship among salivary carbonic anhydrase VI activity and flow rate, biofilm pH and caries in primary dentition. Caries Res. 2012;46(3):194-200. doi: 10.1159/000337275. Epub 2012 Apr 13. PMID: 22508543.
- Picco DCR, Lopes LM, Rocha Marques M, Line SRP, Parisotto TM, Nobre Dos Santos M. Children with a Higher Activity of Carbonic Anhydrase VI in Saliva Are More Likely to Develop Dental Caries. Caries Res. 2017;51(4):394-401. doi: 10.1159/000470849. Epub 2017 Jun 21. PMID: 28633135.
- Esberg A, Haworth S, Brunius C, Lif Holgerson P, Johansson I. Carbonic Anhydrase 6 Gene Variation influences Oral Microbiota Composition and Caries Risk in Swedish adolescents. Sci Rep. 2019 Jan 24;9(1):452. doi: 10.1038/s41598-018-36832-z. PMID: 30679524; PMCID: PMC6345836.
- Baker JL, Edlund A. Exploiting the Oral Microbiome to Prevent Tooth Decay: Has Evolution Already Provided the Best Tools? Front Microbiol. 2019 Jan 11;9:3323. doi: 10.3389/fmicb.2018.03323. PMID: 30687294; PMCID: PMC6338091.
- Szabó I. Carbonic anhydrase activity in the saliva of children and its relation to caries activity. Caries Res. 1974;8(2):187-91. doi: 10.1159/000260107. PMID: 4209465.
- Yan G, Huang W, Xue H, Jia Y, Yang D. [Relationship between dental caries and salivary proteome by electrospray ionization ion-trap tandem mass spectrometry in children aged 6 to 8 years]. Hua Xi Kou Qiang Yi Xue Za Zhi. 2014 Jun;32(3):297-302. Chinese. doi: 10.7518/hxkq.2014.03.020. PMID: 25033650; PMCID: PMC7041220.
- Kivelä J, Parkkila S, Parkkila AK, Rajaniemi H. A low concentration of carbonic anhydrase isoenzyme VI in whole saliva is associated with caries prevalence. Caries Res. 1999 May-Jun;33(3):178-84. doi: 10.1159/000016514. PMID: 10207192.
- Picco DCR, Marangoni-Lopes L, Parisotto TM, Mattos-Graner R, Nobre-Dos-Santos M. Activity of Carbonic Anhydrase VI is Higher in Dental Biofilm of Children with Caries. Int J Mol Sci. 2019 May 30;20(11):2673. doi: 10.3390/ijms20112673. PMID: 31151296; PMCID: PMC6600353.
- Oztürk LK, Furuncuoğlu H, Atala MH, Uluköylü O, Akyüz S, Yarat A. Association between dental-oral health in young adults and salivary glutathione, lipid peroxidation and sialic acid levels and carbonic anhydrase activity. Braz J Med Biol Res. 2008 Nov;41(11):956-9. doi: 10.1590/s0100-879x2008005000048. Epub 2008 Oct 31. PMID: 18982196.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009 Oct;62(10):e1-34. doi: 10.1016/j.jclinepi.2009.06.006. Epub 2009 Jul 23. PMID: 19631507.
- Saaiq M, Ashraf B. Modifying "Pico" Question into "Picos" Model for More Robust and Reproducible Presentation of the Methodology Employed in A Scientific Study. World J Plast Surg. 2017 Sep;6(3):390-392. PMID: 29218294; PMCID: PMC5714990.
- Carvalho, APV, Silva, V, Grande, AJ. Assessment of bias risk from trials randomized by the tool of the Cochrane Collaboration. Diagn Tratamento. 2013;18(1):38-44.
- Viswanathan M, Ansari MT, Berkman ND, et al. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. AHRQ Methods for Effective Health Care. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. http://www.ncbi.nlm.nih.gov/books/NBK91433/. Accessed April 8, 2019.
- Borghi GN, Rodrigues LP, Lopes LM, Parisotto TM, Steiner-Oliveira C, Nobre-Dos-Santos M. Relationship among α amylase and carbonic anhydrase VI in saliva, visible biofilm, and early childhood caries: a longitudinal study. Int J Paediatr Dent. 2017 May;27(3):174-182. doi: 10.1111/ipd.12249. Epub 2016 Jul 19. PMID: 27430359.
- Gao X, Jiang S, Koh D, Hsu CY. Salivary biomarkers for dental caries. Periodontol 2000. 2016 Feb;70(1):128-41. doi: 10.1111/prd.12100. PMID: 26662487.
- Lalloo R, Tadakamadla SK, Kroon J, Tut O, Kularatna S, Boase R, Kapellas K, Gilchrist D, Cobbledick E, Rogers J, Johnson NW. Salivary characteristics and dental caries experience in remote Indigenous children in Australia: a cross-sectional study. BMC Oral Health. 2019 Jan 17;19(1):21. doi: 10.1186/s12903-018-0692-2. PMID: 30654791; PMCID: PMC6337781.
- Edgar, M., Dawes, C., O´Mullane, D. Composition, functions and protective effects. In: Saliva and Oral Health. São Paulo: Santos; 2010.
- Tenovuo J. Antimicrobial function of human saliva--how important is it for oral health? Acta Odontol Scand. 1998 Oct;56(5):250-6. doi: 10.1080/000163598428400. PMID: 9860091.
- Becks H, Wainwright WW. Human Saliva: IX. the Effect of Activation On Salivary Flow1. Journal of Dental Research. 1939;18(5):447-456. doi:10.1177/00220345390180050601
- Leinonen J, Kivelä J, Parkkila S, Parkkila AK, Rajaniemi H. Salivary carbonic anhydrase isoenzyme VI is located in the human enamel pellicle. Caries Res. 1999 May-Jun;33(3):185-90. doi: 10.1159/000016515. PMID: 10207193.
- Culp DJ, Robinson B, Parkkila S, Pan PW, Cash MN, Truong HN, Hussey TW, Gullett SL. Oral colonization by Streptococcus mutans and caries development is reduced upon deletion of carbonic anhydrase VI expression in saliva. Biochim Biophys Acta. 2011 Dec;1812(12):1567-76. doi: 10.1016/j.bbadis.2011.09.006. Epub 2011 Sep 16. PMID: 21945428; PMCID: PMC3205318.
- Singh S, Sharma A, Sood PB, Sood A, Zaidi I, Sinha A. Saliva as a prediction tool for dental caries: An in vivo study. J Oral Biol Craniofac Res. 2015 May-Aug;5(2):59-64. doi: 10.1016/j.jobcr.2015.05.001. Epub 2015 Jun 3. PMID: 26258015; PMCID: PMC4523584.
- Pandey P, Reddy NV, Rao VA, Saxena A, Chaudhary CP. Estimation of salivary flow rate, pH, buffer capacity, calcium, total protein content and total antioxidant capacity in relation to dental caries severity, age and gender. Contemp Clin Dent. 2015 Mar;6(Suppl 1):S65-71. doi: 10.4103/0976-237X.152943. PMID: 25821379; PMCID: PMC4374323.
- Kotwica J, Ciuk MA, Joachimiak E, Rowinski S, Cymborowski B, Bebas P. Carbonic anhydrase activity in the vas deferens of the cotton leafworm - Spodoptera littoralis (Lepidoptera: Noctuidae) controlled by circadian clock. J Physiol Pharmacol. 2006 Nov;57 Suppl 8:107-23. PMID: 17242477.
- Nobre dos Santos M, Melo dos Santos L, Francisco SB, Cury JA. Relationship among dental plaque composition, daily sugar exposure and caries in the primary dentition. Caries Res. 2002 Sep-Oct;36(5):347-52. doi: 10.1159/000065959. PMID: 12399695.
- Parisotto TM, Steiner-Oliveira C, Duque C, Peres RC, Rodrigues LK, Nobre-dos-Santos M. Relationship among microbiological composition and presence of dental plaque, sugar exposure, social factors and different stages of early childhood caries. Arch Oral Biol. 2010 May;55(5):365-73. doi: 10.1016/j.archoralbio.2010.03.005. Epub 2010 Apr 9. PMID: 20381791.