More Information
Submitted: September 19, 2023 | Approved: October 06, 2023 | Published: October 09, 2023
How to cite this article: Mateo Mediavilla I, Collado Yurrita LR, Ciudad MJ, Fernández Domínguez M, López-Píriz R. Laser Doppler Flowmeter as a Periodontal Evaluation Method: A Clinical Pilot Study. J Clin Adv Dent. 2023; 7: 026-033.
DOI: 10.29328/journal.jcad.1001037
Copyright License: © 2023 Mateo Mediavilla I, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Laser Doppler; Gingival blood flow; Periodontal vascularity; Periodontal disease
Laser Doppler Flowmeter as a Periodontal Evaluation Method: A Clinical Pilot Study
Mateo Mediavilla I1,2, Collado Yurrita LR3, Ciudad MJ3, Fernández Domínguez M2,4 and López-Píriz R1,2,5*
1Advance Oral Surgery Institute (ICOA), Madrid, Spain
2Dental School, CEU-San Pablo University, Spain
3Dental School, Complutense University Madrid, Spain
4Oral and Maxillofacial Surgery, Madrid Hospital, Spain
5Nanotechnology and Nanomaterials Research Institute, CINN-CSIC, Spain
*Address for Correspondence: López-Píriz R, Nanotechnology and Nanomaterials Research Institute, CINN-CSIC, Spain, Email: [email protected]
Background and objectives: Periodontal disease, as an inflammatory pathology, induces hemodynamic changes that can be evaluated by different unbiased methods such as laser Doppler flowmetry. This clinical investigation assesses laser Doppler as a non-invasive procedure to monitor gingival vascularization and its potential relationship with the response to treatment of periodontal disease.
Materials & methods: 45 sites of white Spanish patients with active periodontitis undertake a complete periodontal analysis. This included periodontal pathogens identification along with the monitoring of the gingival margin microvascularization using a Doppler laser at the points exhibiting the most periodontal damage. All assessments were performed before and after periodontal combined treatment PCT (scaling, root planing, and antibiotic therapy prescription) (n = 45 sites).
Results: Parameters of periodontal disease showed a positive correlation with pathogen levels. Blood flow readings decreased significantly after PCT (p < 0,05), although this parameter was not statistically correlated with periodontal nor microbial assessments in a significant range.
Conclusion: Laser Doppler is a complementary method of monitoring periodontal inflammation to traditional techniques of clinical periodontal evaluation. Further studies are necessary to determine its usefulness as a predictive method of periodontal disease evolution.
Periodontitis is the main cause of dental loss in the adult population. However, diagnosis of periodontal disease is still based upon subjective clinical examination procedures, which are time-consuming and poorly implemented in general dental practice [1]. Research into periodontal disease is one of the leading topics in dental knowledge. Special attention has been focused on new innovative methods in periodontal diagnosis, specifically built on the momentum of the genomic and proteomic era, as well as advances in cell biology and cell signaling upon periodontal diagnosis and therapy. Considering the essential need for translation of basic research into office practice, and the thorny issue of how cost-effective periodontal therapy is, new approaches in parameters for periodontal diagnosis and treatment monitoring are of paramount relevance.
Periodontal health is defined as the absence of inflammation even in a reduced periodontium. Inflammation constitutes a paramount sign of periodontal disease and the first step in the cellular and humoral immune response [2]. Periodontal pathogens and their invasion of gingival epithelium cells, alter periodontum in two ways: A.- causing tissue destruction; and B.- producing harmful substances that, during tissue damage, act as proinflammatory cytokines that stimulate the inflammatory response [3-6]. Early changes of this defensive reaction occur at a microvascular level as a form of angiogenesis, due to dilatation of the capillaries and an increase in the number of them [7,8]. This can be transferred to both the initial gingivitis and chronic inflammatory periodontal processes.
Several studies employ laser Doppler flowmetry (LDF) as a non-invasive method to assess tissue vascularity. This technique can be used in different microvascular systems such as skin, colonic, muscular, gingival, pulpal, and oral mucosal tissues [9-23]. An animal study measured the influence of irradiation on decreased jaw-bone vascularization, validating LDF to monitor alveolar bone vascularity [24]. The establishment of a normal values range for bone micro-vascularization may reduce the risk of osteoradionecrosis in implant treatment of irradiated patients [25]. Normal blood flow velocity values could be calculated relative to periodontal tissues, as recently reported for eye microvasculature [26]. In Periodontics, LDF appraisals of tissue response to either basic periodontal treatment (scaling and root planning) [27,28] or surgical approach [18,19] have proved laser Doppler readings to be positively correlated to gingival inflammation reduction [28-30]. Other studies that focus on gingival micro-vascularization differences between smokers and non-smokers failed to find a lower gingival blood flow in non-smoking patients with the same degree of periodontitis. Tobacco has not proved to be capable of causing vasoconstriction in oral tissues, contrary to clinical observations in which there is a lower tendency to bleeding on probing in this group of patients [18,21].
Eventually, it could be possible to establish a threshold in periodontal vascularity value beyond which periodontal bone resorption could be triggered. As a matter of fact, impartial clinical decisions in periodontal diagnosis and treatment outcomes might be influenced by assessing micro-vascularity in periodontal tissues. Hence, clinical implementation of laser Doppler readings may increase the predictability of periodontal treatments. However, to prove this method to be useful in human beings, normal values of human periodontal vascularity measured by LDF constitute a pre-requisite. As far as we know, no study has considered how the presence of periodontal pathogens influences gingival blood flow/gingival inflammation. This study tackles the evolution of the gingival blood flow after combined therapy with basic periodontal treatment and antibiotics.
Study design and population
This pilot study includes a total of 9 white Spanish patients (4 women and 5 men) between 26 and 71 years old (mean 44.67, standard deviation SD 13.17). All participants sought periodontal treatment in private practice and exhibited periodontal biotype medium [31] and disease stage III and IV, grade C [32]. It was distinguished between smokers and non-smokers.
The following exclusion criteria were considered: patients with a history of excessive consumption of drugs and/or alcohol, pregnant or breastfeeding, infectious diseases, intravenous bisphosphonates treatment, antibiotic treatment for less than 2 months before starting with the first part of the study, diabetics, chemotherapy, head or neck irradiation, hematologic disorders, as well as in psychiatric patients.
The protocol study complies with the ethical precepts formulated in the Declaration of Helsinki of the World Medical Association on the ethical principles for medical research in human beings and in their subsequent revisions, as well as those requirements for the applicable legal regulations. It was approved by the clinical research ethics committee of the San Carlos Clinical Hospital in Madrid (C.I. 17/129C). All patients signed an informed consent of the medical process and its inclusion in the research study.
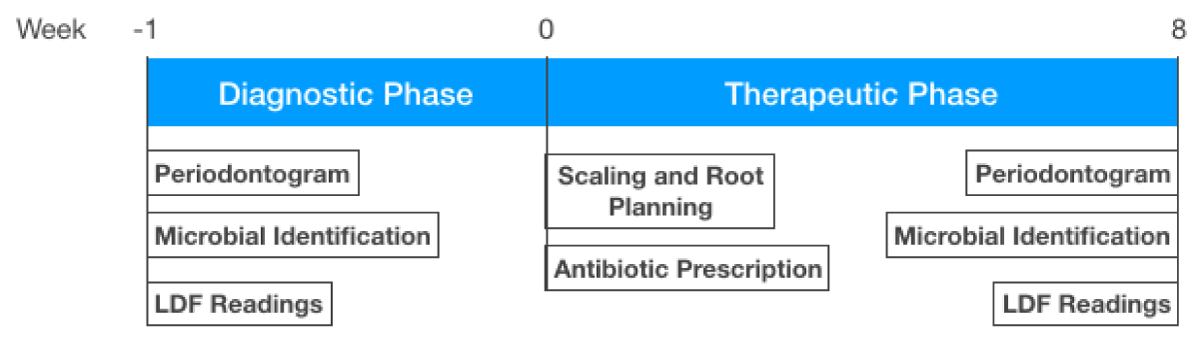
The timeline of the experiment (Figure 1) had three checkpoints:
Figure 1: Timeline of the experiment.Week 1: Diagnostic phase with a periodontal chart fulfilled, microbiological testing of periodontal microorganism, and microvascular blood flow readings using laser Doppler flowmetry. Week 0: Periodontal treatment (scaling, root planing, and antibiotic prescription). Week 8: Periodontal reevaluation (periodontal chart, periodontal pathogen identification, and blood flow readings using laser Doppler flowmetry).
1. Periodontal study which included periodontogram, periodontal pathogens identification, and pre-treatment blood flow laser Doppler readings at the 5 sites exhibiting the greatest periodontal disease activity;
2. One-stage scaling and root planning treatment followed by antibiotic prescription (Metronidazole 500 mg / 12h / 7 days [33,34]),
3. Eight weeks later, all parameters were re-evaluated to assess the correlation between clinical intervention, microbial counts, and LDF readings.
Periodontal evaluation
Clinical data from patients included an exhaustive anamnesis form, orthopantomography (OPG), and standardized periapical radiographic series [35]. The periodontal chart was fulfilled using a CP-12 periodontal probe, and included assessments of probing depth (PD), bleeding on probing (BOP) scored according to the Löe and Silness criteria, plaque index (PI) recorded by the O’Leary plaque index, clinical attachment loss (CAL) and recessions (REC). All these measurements involved six tooth aspects (mesiobuccal, buccal, distobuccal, mesio-lingual, lingual, and disto-lingual) [36-40]. Periodontogram also came with tooth mobility, sulcular suppuration, and furcation defects.
Microbiological testing of periodontal microorganisms
The five sites with higher PD values from periodontal evaluation were allocated for bacterial sample collection. Protocol for sulcular crevicular fluid bacterial samples included supragingival extensive drying and plaque removal with sterile cotton pellets.
The processing of the samples was carried out in the Microbiology and Molecular Virology Unit of the Analysis Laboratories Dr. Echevarne (Barcelona, Spain) within 24 hours. For the identification of periodontal pathogens, in samples of crevicular fluid, a conventional polymerase chain reaction (PCR) was performed followed by identification by reverse hybridization on a colorimetric strip. The microIDent® kit (Hain Lifescience, GmbH, Nehren, Germany) was used, based on DNA · STRIP® technology, which allows the semi-quantitative detection of Aggregatibacter actinomycetemcomitans (Aa), Porphyromonas gingivalis (Pg), Tannerella forsythia (Tf), Treponema denticola (Td) and Prevotella intermedia (Pi).
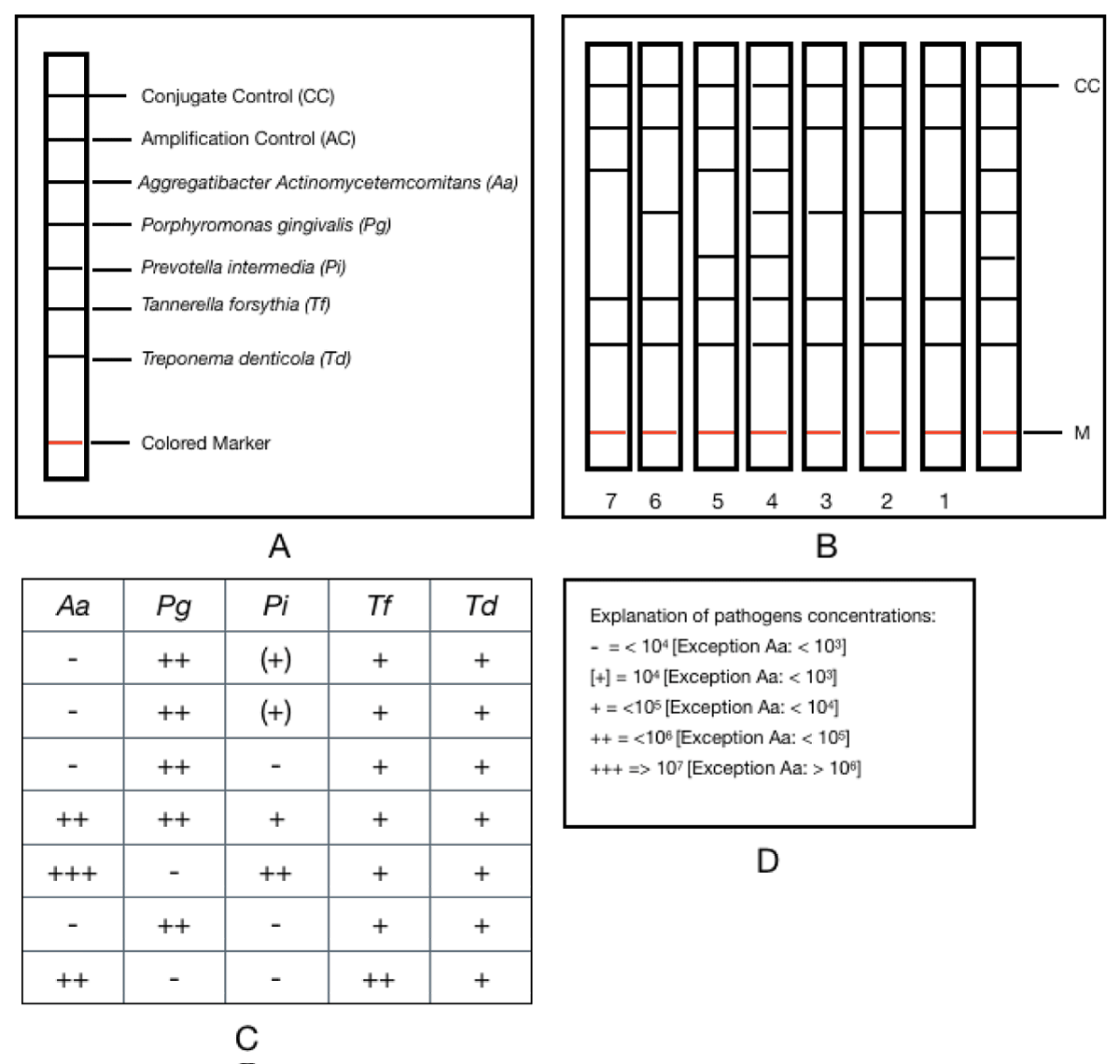
For this, a multiplex PCR was carried out, using complementary oligonucleotides of fraction 16 of the rDNA corresponding to these microorganisms, followed by simultaneous reverse hybridization on a nylon strip with colorimetric labeling. Multiplex amplification was accomplished with biotin-labeled primers. The reverse hybridization procedure was performed according to the instructions of the manufacturer of the microIDent® kit (Hain Lifescience, Nehren, Germany). Each strip supplied by the manufacturer of the microIDent® test has a total of 7 reaction zones: 2 corresponding to quality control (Conjugate Control (CC) and Amplification Control (AC)), and 5 specific bands for each bacterial species (Aa, Pg, Tf, Td and Pi) (Figure 2A). The results were determined according to the intensity of staining of each line in the specific bands, corresponding to each bacterium (Figure 2B). The degree of staining of each band was represented by crosses (Figure 2C), following a scale from lower to higher: (+), +, ++, +++, which the commercial company makes correspond with a specific concentration for each bacterium (Figure 2D). The absence of staining was considered negative, indicating that the sample contained less than the detectable level of nucleic acid for the target microorganism. The detection limit was determined by the manufacturer and corresponded to 103 genomes, in the case of Aa, and 104 genomes for Pg, Pi, Tf, and Td.
Figure 2: PCR results interpretation accord to the microIDent® kit manufacturer (Hain Lifescience, GmbH, Nehren, Germany) A-Reaction zones (sites 1-7). B- Staining of line in the specific bands, corresponding to each bacterium. C- Degree of staining of each band represented by crosses. D- Explanation of pathogens concentrations.
Micro-vascular flow assessments
Laser Doppler Flowmeter Figure 3 (Moor VMS-PC®, Moor Instruments Limited UK) surveyed blood flow at the periodontal gingival margin. The probe used in this experiment displayed a 1.5 mm diameter and a fiber distance of 0.5 mm (VP3 needle-shaped probe) Figure 4. This flowmeter is a semiconductor diode laser with a wavelength of 780 nm, based on the Doppler effect that provides continuous monitoring of blood flow with a sampling frequency of 40 Hz and a depth of 1 mm. Doppler effect consists of the apparent frequency change of a wave produced by the relative movement of a source with respect to an observer. In the blood flow, by striking a beam of light on any human tissue, it is dispersed both by the static structures and by the red blood cells. Those beams returned by the red blood cells suffer a deviation in their frequency that is enlarged, however, those that affect the static structures are not modified. Both light fractions are captured by a photodetector and processed to determine blood flow [24].
Figure 3: Laser Doppler flowmeter monitor Moor VMS-LDF1-HP.
Figure 4: VP3 Needle shape probe.
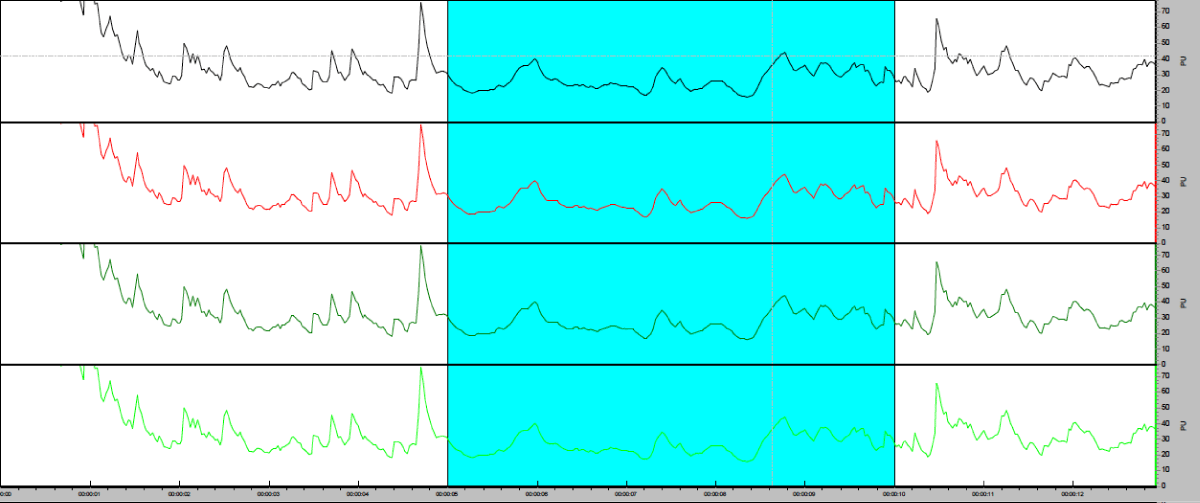
Flowmeters measure blood flow in perfusion units (PU) as the product of the average speed and concentration of blood cells in a single volume of tissue. “Standard Motility” consisting in a low concentration of polystyrene microspheres in water submitted along thermal movement (brownian movement) calibrates the probe. A memory chip in the probe stores figures from the calibration process. Probe positioning on the gingival margin was stabilized by means of a customized splint in order to sustain measurement reproductivity. Splint is important to minimize scattering from structures other than blood cells that could generate Doppler changes and produce an indistinguishable signal from that caused by blood flow itself. LDF readings were recorded during an interval of 20 seconds (Figure 5). Blood flow assessments were performed at each site independently so that positioning in the split did not scatter readings during probe recording.
Figure 5: Blood flow measurements in PU with the Moor-VMS software. 20 seconds selected.
Factors such as room temperature and brightness are confounding factors for LDF readings and may cause bias in results. Since low temperatures (< 15 ºC) reduces significantly Doppler laser flowmeter signal, the room was maintained at all times in a 23 ºC - 26 ºC range during procedures. The dental chair was switched off and exposure from outside light was controlled to minimize probe scattering from external light energy sources.
Statistical analysis
Statistics software SPSS® was used to perform data analysis. All measurements were described by means and SD, statistical significance was pointed for a p < 0.05. Blood flow was considered a dependent variable and compared with indirect variables such as gender, age, toxic habits (smoke), PD, BOP, PI, CAL, and periodontal pathogens presence. Measurement values at different timeline points were analyzed by the T-Student test for paired samples, using the range with Wilcoxon signs when appropriate. Pearson correlation coefficient was used to determine the correlation between blood flow (PU) and the rest of the parameters. Differences between subjects (smoker or not) were compared by means of the Mann-Whitney U test.
Demographic characteristics and blood flow readings (PU)
LDF readings mean at T1 (week -1) was 86.85 SD of 103.44. After PCT, LDF readings were significantly lower (mean 54.31 and SD 52.82), being statistically significant (p = 0.048, mean -32.50, SD 107.32).
Data analysis revealed significantly lower PU in female patients compared with male patients at T1(week -1) and at T2 (week 8). T-Student Test showed also a decrease in PU values statistically significant in female patients (p < 0.05) after PCT.
Pearson’s correlation test indicates a statistical signification comparing microvascular readings with age, younger patients exhibited bigger difference in LDF readings after PTC than older ones (p = 0.016).
No statistical differences were found between smokers or not in microvascular readings previous or after periodontal treatment (Mann-Withney U test p > 0.05) (Table 1).
| Table 1: Demographic characteristics and blood flow readings were measured in perfusion units. Means, standard deviations, and p values. | |||||||||||||||
| Perfusion Units | Global population | p value (AGE) |
Men | Women | p value | Smokers | Non smokers | pvalue | |||||||
| Mean | SD | Mean | SD | p value | Mean | SD | p value | Mean | SD | Mean | SD | ||||
| T1.Week -1 | 86.85 | 103.44 | .023 | 119.37 | 142.38 | 60.83 | 44.51 | .024 | 67.18 | 46.82 | 102.58 | 131.53 | .694 | ||
| T2.Week 8 | 54.31 | 52.82 | .679 | 72.02 | 70.53 | 40.20 | 26.57 | .107 | 47.19 | 35.96 | 60.06 | 63.35 | .253 | ||
| Interventional Change |
-32.50 | 107.32 | .016 | -47.35 | 151.93 | .179 | -20.62 | 50.13 | .05 | -19.98 | 50.24 | -42.52 | 137.42 | .607 | |
Periodontal parameters and blood flow readings (PU)
Men had worse indicators of periodontal health than women, even this was not statistically significant. Eight weeks after PCT, all patients had improvements in all periodontal parameters, in terms of pocket reduction, gain of clinical attachment level and reduction in full mouth bleeding and plaque scores. Probing depth decreased mean -2.08, SD 1.51 p < 0.05. The full mouth bleeding on probing percentage was 65% in week -1 and after PCT (week 8) was 27.44%. In addition, the plaque index was significantly reduced and the mean percentage of plaque in the whole mouth went from 79% to 48% after PCT. Regarding CAL, it experienced an average improvement of -2.08 SD 1.51. Pearson’s correlation tests did not show a significant correlation with the decrease in LDF (p > 0.05) (Table 2).
| Table 2: Periodontal parameters correlate with blood flow readings measured in perfusion units. Means, standard deviations, and p values. | ||||||||||||||||
| Periodontal Parameters | PD | p Value (PU) | CAL | p value (PU) | PI | PU | BOP | p value (PU) | ||||||||
| Mean | SD | p Value | Mean | SD | p value | Mean | SD | P value | Mean | SD | p value | |||||
| T1.Week -1 | 6.27 | 1.30 | - | .935 | 6.64 | 1.78 | - | .873 | 79 % | 18.52 | - | .914 | 65 % | 20.79 | - | .123 |
| T2. Week 8 | 4.2 | 1.07 | - | .170 | 4.56 | 1.63 | - | .983 | 48 % | 30.57 | - | .990 | 27.44% | 10.34 | - | .615 |
| Interventional Change |
-2.08 | 1.51 | .000 | .905 | -2.067 | 1.09 | .000 | .699 | -31 % | 12,05 | .000 | - | -37,56 % | 10,45 | .000 | - |
Periodontal pathogens count and periodontal and microvascular parameters
Pearson’s correlation test showed a significant reduction in all periodontal pathogens (p = 0.000) after PCT, except for the reduction in Td, which was not statistically significant.
A positive relationship could be established between certain pathogen reductions and PU levels, as well as plaque index and bleeding on the probing index (Table 3).
| Table 3: Periodontal pathogens correlated with periodontal and microvascular parameters in T1 and T2 (Mann Whitney U test < 0.05 p values). | ||||||||||
| Periodontal and Microvascular Parameters | Aa | Pg | Tf | Td | Pi | |||||
| T1 Week -1 | T2 Week 8 | T1 Week -1 | T2 Week 8 | T1 Week -1 | T2 Week 8 | T1 Week -1 | T2 Week 8 | T1 Week -1 | T2 Week 8 | |
| PU | 0.05 | - | - | - | - | .028 | .045 | - | .049 | - |
| PD | - | - | - | - | - | - | - | - | .006 | - |
| BOP | - | - | .015 | - | .000 | .000 | - | .05 | .045 | .006 |
| IP | - | - | - | - | - | .001 | .000 | .000 | .000 | - |
| CAL | - | - | - | - | - | - | - | .000 | .004 | - |
| DIFF PU | .05 | - | - | - | .047 | - | - | - | - | - |
Until today, the most used methods for evaluation and monitoring periodontal disease are indirect procedures based on clinical evaluations and determinations of the subgingival microflora. The direct methods used previously required invasive techniques such as biopsies, which cause an irreversible change in the tissues studied [41].
Several studies in animals have shown that blood flow is greater in an inflamed gingiva than in a healthy one due to blood stasis [42,43]. Likewise, when gingival inflammation occurs in humans, changes are detected in the marginal gingiva, consisting of an increase in the number of visible vessels, among others [44]. These changes never reverted back to the original blood flow healthy pattern even when inflammation had resolved [45]. Therefore, the use of blood flow as a predictive indicator of future healing and prognosis of periodontal disease is a concept worth pursuing [46].
In the present experiment, the blood flow in the marginal gingiva was notably greater before PCT than 8 weeks after it, having produced both a clinical and statistical reduction in the evaluation parameters of gingival inflammation and periodontal status, considering in some cases the site studied without gingival inflammation (no BOP, PD£3mm). The classic diagnostic parameters of PD, BOP, and CAL, as well as their effectiveness in the monitoring of periodontal disease, have been widely described. It was possible to establish a positive correlation between these parameters and gingival blood flow in the marginal and intracellular gingiva at the disease sites compared to healthy ones. This relationship is also directly proportional to the severity of periodontitis, increasing with greater severity [27,47]. In this study, we also have found a positive relationship, although not statistically significant, between the classic parameters to evaluate periodontal disease and blood flow, the analysis of the data shows a significant decrease after treatment in all parameters, both periodontal and flow of blood.
A possible limitation in the data collection process was the size of the probe. The gingival LDF signal is dominated by the flow mainly from the superficial vessels, so with the current wavelength of the laser light and the construction of the probe, it did not allow us to obtain records of the blood flow within the periodontal pocket, where the first inflammatory changes occur and the largest number of pathogens are present.
All patients included had established stage III and IV and grade C periodontitis, but it would be interesting to focus future research on the evolution of blood flow in relation to inflammation development since greater readings have been described in chronic periodontitis than in the initial gingivitis [28].
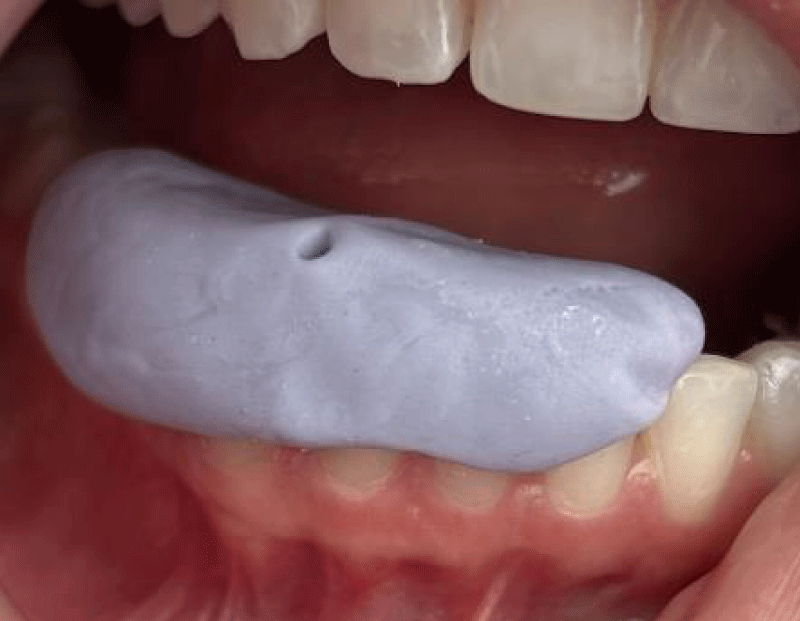
Probe splinting or manual blood flow measurement was a controversial issue in previous studies [28,48]. It has been reported higher measure values when performed by hand than splinting, which may be due to the pressure of the splinting material that compresses the gingiva. For this reason, in this study, the measurements were always carried out by the same operator with a customized splint, seeking not to interfere with the natural vascularization conditions by not contacting the gingival tissue and avoiding previously performed injections of anesthesia with a vasoconstrictor Figure 6.
Figure 6: Customized splint.
Comparison with previous studies was hampered by the variety of Doppler lasers used with different calibration constants and units of measurement, as well as by the different study protocols. Even so, the minimum time required to measure blood flow was established, in line with these studies, at 20 consecutive seconds per monitored site [21,49-51].
Other studies based on microvascular flow assessments, employed different methods such as Optical Coherence Technology Angiography (OCTA) [52]. This technique, although non-invasive, is also limited by its expensiveness, slow acquisition time, and small field of imaging among others [53]. Fluorescein angiography (FA) has considerable shortcomings, such as being invasive, having a long image acquisition time, and not providing quantitative data. Also, near-infrared spectroscopy provides deeper tissue information on blood flow but it’s not vessel-specific [54].
LDF is a proven monitoring tool of vascularization in other human tissues as alveolar bone [25]. The main application will be evaluating the osseointegration of dental implants, making microvascularization a crucial factor in implant stability. Human besides animal studies showed that LDF is an adequate method for bone microvascularity evaluation and might determine future implant success [55]. Therefore, laser Doppler flowmetry seemed to be a valid option in the search for new direct and non-invasive methods of microvascular flow measurements.
One of the factors that influence the progression of periodontal disease is the combined action of related pathogens, capable of causing changes in the total oral microbiota, altering the homeostatic balance of the tissue [55-58]. Above all, the presence of the Socransky red complex bacteria (dominant in the late stages of plaque development and mainly found in cases of periodontitis in adults) is strongly associated with parameters of periodontal inflammation, including PD and BOP [56,59]. The presence of these pathogenic species has also been detected in healthy individuals, so for periodontal disease to manifest itself clinically, in addition to a subgingival microbiota shift (favoring excessive growth of pathogenic species), a suitable environment and susceptible host are essentiall [57,60].
Recent studies have focused on the role of innate immunity in the pathogenesis of periodontitis [61]. The final immune response occurs at the local level, being essential for microbiota regulation, by stimulating microenvironmental changes and limiting the increase in periodontal pathogens that help the most protective bacteria to predominate [62]. Despite the fact that, in this study, the relationship between the decrease in periodontopathogenic species in the studied sites and the evolution of blood flow and inflammation parameters appears to be positive, it lacks sufficient statistical significance. This supports the theory that, although the role of periodontopathogenic bacteria in the development of periodontal disease is undeniable, it is not exclusively an infectious but an inflammatory pathology, with an essential role in the host’s immune system.
Since the exact mechanisms of the pathogenesis of periodontitis remain unclear, and multiple factors can destabilize this balance contributing to the pathogenesis of periodontitis, the counterbalance of different diagnostic parameters results in challenging for such a multifactorial disease.
In light of the findings of this study, LDF is a feasible method for gingival vascularization assessments, which in combination with traditional periodontal parameters, provides useful information for periodontal disease diagnosis and treatment monitoring.
Especially in the most clinically affected sites, a reduction in the means of blood flow readings was detected, as well as the correlation between the parameters of the evaluation of periodontal disease and the number of pathogens found.
Further studies are necessary to focus on its usefulness as a predictive method of the evolution of periodontal disease with a larger sample size and an increased follow-up time.
- Morgan RG. Quality evaluation of clinical records of a group of general dental practitioners entering a quality assurance programme. Br Dent J. 2001 Oct 27;191(8):436-41. doi: 10.1038/sj.bdj.4801201. PMID: 11720017.
- Lang NP, Bartold PM. Periodontal health. J Periodontol. 2018 Jun;89 Suppl 1:S9-S16. doi: 10.1002/JPER.16-0517. PMID: 29926938.
- Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis. Periodontol 2000. 1997 Jun;14:12-32. doi: 10.1111/j.1600-0757.1997.tb00190.x. PMID: 9567964.
- Deshpande RG, Khan MB, Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998 Nov;66(11):5337-43. doi: 10.1128/IAI.66.11.5337-5343.1998. PMID: 9784541; PMCID: PMC108667.
- Taichman N, Lindhe J. Pathogenesis of plaque-associated periodontal disease. In: Textbook of clinical periodontology. 2nd ed. Copenhagen, Denmark: Munksgaard International Publishers. 1992; 153– 192.
- Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012 Sep;249(1):158-75. doi: 10.1111/j.1600-065X.2012.01146.x. PMID: 22889221; PMCID: PMC3662247.
- Zoellner H, Hunter N. Vascular expansion in chronic periodontitis. J Oral Pathol Med. 1991 Oct;20(9):433-7. doi: 10.1111/j.1600-0714.1991.tb00434.x. PMID: 1725185.
- Egelberg J. The blood vessels of the dento-gingival junction. J Periodontal Res. 1966;1(3):163-79. doi: 10.1111/j.1600-0765.1966.tb01857.x. PMID: 4225527.
- Holloway GA Jr, Watkins DW. Laser Doppler measurement of cutaneous blood flow. J Invest Dermatol. 1977 Sep;69(3):306-9. doi: 10.1111/1523-1747.ep12507665. PMID: 894068.
- Seike K, Koda K, Saito N, Oda K, Kosugi C, Shimizu K, Miyazaki M. Laser Doppler assessment of the influence of division at the root of the inferior mesenteric artery on anastomotic blood flow in rectosigmoid cancer surgery. Int J Colorectal Dis. 2007 Jun;22(6):689-97. doi: 10.1007/s00384-006-0221-7. Epub 2006 Nov 3. PMID: 17082922.
- Røe C, Damsgård E, Knardahl S. Reliability of bloodflux measurements from the upper trapezius muscle during muscle contractions. Eur J Appl Physiol. 2008 Mar;102(5):497-503. doi: 10.1007/s00421-007-0610-9. Epub 2007 Nov 15. PMID: 18004589.
- Kocabalkan E, Turgut M. Variation in blood flow of supporting tissue during use of mandibular complete dentures with hard acrylic resin base and soft relining: a preliminary study. Int J Prosthodont. 2005 May-Jun;18(3):210-3. PMID: 15945307.
- von Arx T, Chappuis V, Winzap-Kälin C, Bornstein MM. Laser Doppler flowmetry for assessment of anterior mandibular teeth in conjunction with bone harvesting in the symphysis: a clinical pilot study. Int J Oral Maxillofac Implants. 2007 May-Jun;22(3):383-9. PMID: 17622004.
- Emshoff R, Kranewitter R, Brunold S, Laimer K, Norer B. Characteristics of pulpal blood flow levels associated with non-segmented and segmented Le Fort I osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008 Mar;105(3):379-84. doi: 10.1016/j.tripleo.2007.08.027. PMID: 18280970.
- Ubbink DT. Toe blood pressure measurements in patients suspected of leg ischaemia: a new laser Doppler device compared with photoplethysmography. Eur J Vasc Endovasc Surg. 2004 Jun;27(6):629-34. doi: 10.1016/j.ejvs.2004.01.031. PMID: 15121114.
- Trapp LD, Goodson JM, Price DC. Evaluation of oral submucosal blood flow at dental injection sites by radioactive Xenon clearance in beagle dogs. J Dent Res. 1977 Aug;56(8):889-93. doi: 10.1177/00220345770560080801. PMID: 270486.
- Xiang X, Sowa MG, Iacopino AM, Maev RG, Hewko MD, Man A, Liu KZ. An update on novel non-invasive approaches for periodontal diagnosis. J Periodontol. 2010 Feb;81(2):186-98. doi: 10.1902/jop.2009.090419. PMID: 20151796.
- Mavropoulos A, Brodin P, Rösing CK, Aass AM, Aars H. Gingival blood flow in periodontitis patients before and after periodontal surgery assessed in smokers and non-smokers. J Periodontol. 2007 Sep;78(9):1774-82. doi: 10.1902/jop.2007.060472. PMID: 17760548.
- Rajan V, Varghese B, van Leeuwen TG, Steenbergen W. Review of methodological developments in laser Doppler flowmetry. Lasers Med Sci. 2009 Mar;24(2):269-83. doi: 10.1007/s10103-007-0524-0. Epub 2008 Jan 31. PMID: 18236103.
- Singh DB, Stansby G, Harrison DK. Assessment of oxygenation and perfusion in the tongue and oral mucosa by visible spectrophotometry and laser Doppler flowmetry in healthy subjects. Adv Exp Med Biol. 2008;614:227-33. doi: 10.1007/978-0-387-74911-2_26. PMID: 18290333.
- Meekin TN, Wilson RF, Scott DA, Ide M, Palmer RM. Laser Doppler flowmeter measurement of relative gingival and forehead skin blood flow in light and heavy smokers during and after smoking. J Clin Periodontol. 2000 Apr;27(4):236-42. doi: 10.1034/j.1600-051x.2000.027004236.x. PMID: 10783836.
- Ingólfsson AR, Tronstad L, Hersh EV, Riva CE. Efficacy of laser Doppler flowmetry in determining pulp vitality of human teeth. Endod Dent Traumatol. 1994 Apr;10(2):83-7. doi: 10.1111/j.1600-9657.1994.tb00065.x. PMID: 8062812.
- Ketabi M, Hirsch RS. The effects of local anesthetic containing adrenaline on gingival blood flow in smokers and non-smokers. J Clin Periodontol. 1997 Dec;24(12):888-92. doi: 10.1111/j.1600-051x.1997.tb01207.x. PMID: 9442425.
- Verdonck HW, Meijer GJ, Laurin T, Nieman FH, Stoll C, Riediger D, Stoelinga PJ, de Baat C. Assessment of vascularity in irradiated and nonirradiated maxillary and mandibular minipig alveolar bone using laser doppler flowmetry. Int J Oral Maxillofac Implants. 2007 Sep-Oct;22(5):774-8. PMID: 17974112.
- Verdonck HW, Meijer GJ, Kessler P, Nieman FH, de Baat C, Stoelinga PJ. Assessment of bone vascularity in the anterior mandible using laser Doppler flowmetry. Clin Oral Implants Res. 2009 Feb;20(2):140-4. doi: 10.1111/j.1600-0501.2008.01631.x. Epub 2008 Dec 1. PMID: 19077149.
- Koutsiaris AG, Riri K, Boutlas S, Daniil Z, Tsironi EE. A normative blood velocity model in the exchange microvessels for discriminating health from disease: Healthy controls versus COVID-19 cases. Clin Hemorheol Microcirc. 2023;84(2):215-226. doi: 10.3233/CH-231780. PMID: 37182862.
- Hinrichs JE, Jarzembinski C, Hardie N, Aeppli D. Intrasulcular laser Doppler readings before and after root planing. J Clin Periodontol. 1995 Nov;22(11):817-23. doi: 10.1111/j.1600-051x.1995.tb01778.x. PMID: 8550856.
- Gleissner C, Kempski O, Peylo S, Glatzel JH, Willershausen B. Local gingival blood flow at healthy and inflamed sites measured by laser Doppler flowmetry. J Periodontol. 2006 Oct;77(10):1762-71. doi: 10.1902/jop.2006.050194. PMID: 17032121.
- Donos N, D'Aiuto F, Retzepi M, Tonetti M. Evaluation of gingival blood flow by the use of laser Doppler flowmetry following periodontal surgery. A pilot study. J Periodontal Res. 2005 Apr;40(2):129-37. doi: 10.1111/j.1600-0765.2005.00777.x. PMID: 15733147.
- Retzepi M, Tonetti M, Donos N. Gingival blood flow changes following periodontal access flap surgery using laser Doppler flowmetry. J Clin Periodontol. 2007 May;34(5):437-43. doi: 10.1111/j.1600-051X.2007.01062.x. PMID: 17448047.
- Fischer KR, Künzlberger A, Donos N, Fickl S, Friedmann A. Gingival biotype revisited-novel classification and assessment tool. Clin Oral Investig. 2018 Jan;22(1):443-448. doi: 10.1007/s00784-017-2131-1. Epub 2017 May 27. PMID: 28551728.
- Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol. 2018 Jun;89 Suppl 1:S159-S172. doi: 10.1002/JPER.18-0006. Erratum in: J Periodontol. 2018 Dec;89(12):1475. PMID: 29926952.
- Berglundh T, Krok L, Liljenberg B, Westfelt E, Serino G, Lindhe J. The use of metronidazole and amoxicillin in the treatment of advanced periodontal disease. A prospective, controlled clinical trial. J Clin Periodontol. 1998 May;25(5):354-62. doi: 10.1111/j.1600-051x.1998.tb02455.x. PMID: 9650870.
- Winkel EG, Van Winkelhoff AJ, Timmerman MF, Van der Velden U, Van der Weijden GA. Amoxicillin plus metronidazole in the treatment of adult periodontitis patients. A double-blind placebo-controlled study. J Clin Periodontol. 2001 Apr;28(4):296-305. doi: 10.1034/j.1600-051x.2001.028004296.x. PMID: 11314884.
- Mol A. Imaging methods in periodontology. Periodontol 2000. 2004;34:34-48. doi: 10.1046/j.0906-6713.2003.003423.x. PMID: 14717854.
- O'Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972 Jan;43(1):38. doi: 10.1902/jop.1972.43.1.38. PMID: 4500182.
- Lang NP, Joss A, Orsanic T, Gusberti FA, Siegrist BE. Bleeding on probing. A predictor for the progression of periodontal disease? J Clin Periodontol. 1986 Jul;13(6):590-6. doi: 10.1111/j.1600-051x.1986.tb00852.x. PMID: 3489010.
- Lang NP, Adler R, Joss A, Nyman S. Absence of bleeding on probing. An indicator of periodontal stability. J Clin Periodontol. 1990 Nov;17(10):714-21. doi: 10.1111/j.1600-051x.1990.tb01059.x. PMID: 2262585.
- Greenstein G. The role of bleeding upon probing in the diagnosis of periodontal disease. A literature review. J Periodontol. 1984 Dec;55(12):684-8. doi: 10.1902/jop.1984.55.12.684. PMID: 6394735.
- Lang NP, Joss A, Tonetti MS. Monitoring disease during supportive periodontal treatment by bleeding on probing. Periodontol 2000. 1996 Oct;12:44-8. doi: 10.1111/j.1600-0757.1996.tb00080.x. PMID: 9567993.
- Liljenberg B, Lindhe J. Juvenile periodontitis. Some microbiological, histopathological and clinical characteristics. J Clin Periodontol. 1980 Feb;7(1):48-61. doi: 10.1111/j.1600-051x.1980.tb01948.x. PMID: 6928856.
- Firkova E, Bouka M. Laser Doppler Flowmetry in the Evaluation of Periodontal Health and Disease. J of IMAB. 2019; 25(3):2599-2602. https://doi.org/10.5272/jimab.2019253.2599
- Kaplan ML, Jeffcoat MK, Goldhaber P. Blood flow in gingiva and alveolar bone in beagles with periodontal disease. J Periodontal Res. 1982 Jul;17(4):384-9. doi: 10.1111/j.1600-0765.1982.tb01169.x. PMID: 6217318.
- Matheny JL, Abrams H, Johnson DT, Roth GI. Microcirculatory dynamics in experimental human gingivitis. J Clin Periodontol. 1993 Sep;20(8):578-83. doi: 10.1111/j.1600-051x.1993.tb00774.x. PMID: 8408719.
- Hock J, Niki K. A vital microscopy study of the morphology of normal and inflamed gingiva. J Periodontal Res. 1971;6(2):81-8. doi: 10.1111/j.1600-0765.1971.tb00592.x. PMID: 4255225.
- Krastev B, Filipov I. Laser Doppler Assessment of Microcirculation of Apical Periodontitis before Nonsurgical and during Surgical Laser Treatment. Folia Med (Plovdiv). 2020 Sep 30;62(3):619-625. doi: 10.3897/folmed.62.e48340. PMID: 33009753.
- Vág J, Fazekas A. Influence of restorative manipulations on the blood perfusion of human marginal gingiva as measured by laser Doppler flowmetry. J Oral Rehabil. 2002 Jan;29(1):52-7. doi: 10.1046/j.1365-2842.2002.00818.x. PMID: 11844032.
- Hock JM, Kim S. Blood flow in healed and inflamed periodontal tissues of dogs. J Periodontal Res. 1987 Jan;22(1):1-5. doi: 10.1111/j.1600-0765.1987.tb01532.x. PMID: 2950222.
- Baab DA, Oberg PA, Holloway GA. Gingival blood flow measured with a laser Doppler flowmeter. J Periodontal Res. 1986 Jan;21(1):73-85. doi: 10.1111/j.1600-0765.1986.tb01440.x. PMID: 2937897.
- Mavropoulos A, Aars H, Brodin P. The acute effects of smokeless tobacco (snuff) on gingival blood flow in man. J Periodontal Res. 2001 Aug;36(4):221-6. doi: 10.1034/j.1600-0765.2001.036004221.x. PMID: 11519694.
- Patiño-Marín N, Martínez F, Loyola-Rodríguez JP, Tenorio-Govea E, Brito-Orta MD, Rodríguez-Martínez M. A novel procedure for evaluating gingival perfusion status using laser-Doppler flowmetry. J Clin Periodontol. 2005 Mar;32(3):231-7. doi: 10.1111/j.1600-051X.2005.00655.x. PMID: 15766364.
- Koutsiaris AG, Batis V, Liakopoulou G, Tachmitzi SV, Detorakis ET, Tsironi EE. Optical Coherence Tomography Angiography (OCTA) of the eye: A review on basic principles, advantages, disadvantages and device specifications. Clin Hemorheol Microcirc. 2023;83(3):247-271. doi: 10.3233/CH-221634. PMID: 36502308.
- Khadamy J. Optical Coherence Tomography Angiography (OCTA) in Ophthalmology; Technology, Pros, Cons and Commercial Prototypes. JOJ Ophthal. 2017; 2(5): 555598. DOI: 10.19080/JOJO.2017.02.555598
- Koutsiaris AG. Deep tissue near infrared second derivative spectrophotometry for the assessment of claudication in peripheral arterial disease. Clin Hemorheol Microcirc. 2017;65(3):275-284. doi: 10.3233/CH-16181. PMID: 27983543.
- Vasovic M,Jovanovic L, Djordjevic A. Bone Quality Assessment of Dental Implant Recipient Sites. Experimental and Applied Biomedical Research (EABR). 2022;23(1): 83-87. https://doi.org/10.1515/sjecr-2015-0052
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998 Feb;25(2):134-44. doi: 10.1111/j.1600-051x.1998.tb02419.x. PMID: 9495612.
- Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a potential community activist for disease. J Dent Res. 2012 Sep;91(9):816-20. doi: 10.1177/0022034512453589. Epub 2012 Jul 6. PMID: 22772362; PMCID: PMC3420389.
- Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994 Jun;5:78-111. doi: 10.1111/j.1600-0757.1994.tb00020.x. PMID: 9673164.
- Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12-55. doi: 10.1034/j.1600-0757.2002.280102.x. PMID: 12013340.
- Cullinan MP, Hamlet SM, Westerman B, Palmer JE, Faddy MJ, Seymour GJ. Acquisition and loss of Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans and Prevotella intermedia over a 5-year period: effect of a triclosan/copolymer dentifrice. J Clin Periodontol. 2003 Jun;30(6):532-41. doi: 10.1034/j.1600-051x.2003.00292.x. PMID: 12795792.
- Preshaw PM, Taylor JJ. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol. 2011 Mar;38 Suppl 11:60-84. doi: 10.1111/j.1600-051X.2010.01671.x. PMID: 21323705.
- Ebersole JL, Dawson DR 3rd, Morford LA, Peyyala R, Miller CS, Gonzaléz OA. Periodontal disease immunology: 'double indemnity' in protecting the host. Periodontol 2000. 2013 Jun;62(1):163-202. doi: 10.1111/prd.12005. PMID: 23574466; PMCID: PMC4131201.