More Information
Submitted: November 20, 2025 | Approved: November 25, 2025 | Published: November 26, 2025
How to cite this article: Chickanna R, et al. Evaluation of 2% Bromelain gel and 0.2% Chlorhexidine Gel as Subgingival Local Drug Delivery following Scaling and Root Planing in Stage II / III and Grade B Periodontitis - Randomized Controlled Clinical Trial. J Clin Adv Dent. 2025; 9(1): 007-014. Available from:
https://dx.doi.org/10.29328/journal.jcad.1001050
DOI: 10.29328/journal.jcad.1001050
Copyright license: © 2025 Chickanna R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Periodontitis (specifically Stage II / III and Grade B Periodontitis); 2% Bromelain gel; 0.2% Chlorhexidine gel; Scaling and Root Planing (SRP); Local Drug Delivery (LDD); Randomized controlled clinical trial; Subgingival; Nonsurgical periodontal therapy; Adjunctive therapy; Clinical parameters
Evaluation of 2% Bromelain gel and 0.2% Chlorhexidine Gel as Subgingival Local Drug Delivery following Scaling and Root Planing in Stage II / III and Grade B Periodontitis - Randomized Controlled Clinical Trial
Nallagatta Vamsi Venkatakrishna Sai1, Rudrakshi Chickanna2* and MLV Prabhuji3
1Post Graduate Student, Department of Periodontics, Krishnadevaraya College of Dental Sciences and Hospital, Bengaluru, India
2Reader, Department of Periodontics, Krishnadevaraya College of Dental Sciences and Hospital, Bengaluru, India
3Professor and Head, Department of Periodontics, Krishnadevaraya College of Dental Sciences and Hospital, Bengaluru, India
*Address for Correspondence: Dr. Rudrakshi Chickanna, Department of Periodontics,Krishnadevaraya College of Dental Sciences and Hospital,Bengaluru, Karntaka, India
Background and objectives: Nonsurgical periodontal therapy is the backbone of oral health. The present randomized clinical trial includes the preparation and placement of a herbal medicament adjunctive for periodontitis. Our study includes a comparative evaluation of 2% Bromelain gel with 0.2% Chlorhexidine (CHX) gel as Subgingival Local Drug Delivery (LDD) following Scaling and Root Planing (SRP) in Stage II / III and Grade B Periodontitis.
Methods: Preparation of 2% Bromelain gel and 0.2% Chlorhexidine gel was executed at the Department of Biotechnology, Sir M. Visvesvaraya Institute of Technology, Bengaluru, Karnataka 562157, India. Prepared gels were evaluated in vitro, and the Concentration of Bromelain was set at 2% and Chlorhexidine (CHX) at 0.2%. Clinically, Patients with Stage II / III and Grade B periodontitis underwent scaling and root planning (SRP) and were divided into 3 groups. Randomisation and allocation were done as probing sites receiving only SRP, SRP with adjunct 2% Bromelain gel, and SRP with 0.2% Chlorhexidine gel as groups, respectively. Clinical parameters were assessed at baseline following SRP, at the end of 1 month, and at 3 months. Values obtained were subjected to statistical analysis (p < 0.05).
Results: All the groups show a reduction in all the clinical parameters. Reduction was significantly noted in sites receiving 2% Bromelain gel and 0.2% Chlorhexidine gel adjunct than only SRP. 2% Bromelain gel showed a much greater reduction in inflammation at the end of 1month than 0.2% Chlorhexidine gel. Correlation of clinical variables with time interval showed positive correlation for Gingival Index and modified Sulcular Bleeding Index for the Bromelain group.
Conclusion: Within the scope of this study, Local Drug Delivery using 2% Bromelain and 0.2% Chlorhexidine gel as adjuncts to Scaling and Root Planning demonstrated notable early clinical improvements in the treatment of Stage II/III and Grade B periodontitis.
It is well-established that periodontal disease, based on disease severity and risk factors associated [1,2], arises from bacterial infection involving pathogenic microorganisms within the periodontal pocket. The microbial composition in periodontitis is complex, consisting predominantly of gram-negative anaerobic bacteria [3].
Attempts to suppress dysbiotic bacterial bio load have paved the way for many modalities of periodontal therapy. Fundamental Periodontal treatment remains Scaling and Root Planing (SRP). SRP typically involves mechanical debridement aimed at disrupting the subgingival microbial community and creating root surfaces that are clean, smooth, and biologically compatible. However, the complex structure of the root and the shape of periodontal lesions can sometimes hinder the effectiveness of this approach, making it difficult to sufficiently lower the bacterial load to a biologically acceptable level. Additionally, maintaining control over supragingival plaque is crucial to prevent the re-establishment of periodontal pathogens in the subgingival area [4]. Therefore, it is advocated for adjunctive use of antimicrobial therapy as a part of phase I therapy.
Periodontal disease can be localized or generalized. Site specificity and susceptibility of the individuals necessitate a personalized approach towards periodontal treatments. Use of therapeutic drugs/agents at such targeted sites has been shown to have a beneficial effect by achieving higher concentrations of the drug at the diseased site in the form of sustained or controlled delivery of the drug, i.e., Local Drug Delivery (LDD). This approach overcomes the shortcomings of systemic administration of the drug. Incidence and prevalence of periodontal disease have directed the administration of the drug directly into the pocket as an effective strategy [5].
Years of documented research have established that Chlorhexidine di gluconate (CHX), as the gold standard of chemical plaque control agents, is safe, stable, and effective in preventing and controlling plaque formation [6]. Chlorhexidine (CHX) remains one of the most effective local antimicrobial agents widely used for the local treatment of chronic periodontitis. Chlorhexidine can bind to both skin and mucous membranes. Through the rapid attraction of the negatively charged bacterial cell surface to the cationic CHX molecule, CHX shows strong antibacterial activity in the periodontal pocket [7]. Its mechanism of action is through decreasing pellicle formation, alteration of bacterial adhesion to the tooth surface, alteration of bacterial cell wall, ultimately leading to cell destruction [8].
The use of herbal remedies (Phytotherapy) and products for managing gingivitis, periodontitis, and dental caries has become increasingly popular among both dental professionals and patients. Plant being alkaline in nature, the antibacterial properties of medicinal plants prevents plaque and calculus formation by maintaining acid-alkali balance in saliva [9]. Herbal medicine encompasses herbs, plant-based materials, preparations, and products that contain plant parts or other botanical substances as their active components [10].
Bromelain is one such bioactive compound obtained from the stem and fruit of the pineapple plant [11,12], an extract derived from Ananas comosus, that contains proteinases that exhibit anti-inflammatory properties [13]. Studies have shown efficacy similar to standard Non-Steroidal Anti-Inflammatory drugs (NSAIDs). Furthermore, bromelain presents a large variety of activities such as anti-inflammatory properties in endometriosis, reduction of the neutrophil migration to sites of inflammation, and antibacterial effect against periodontopathogens [14].
Literature search to date has shown very few in-vivo studies regarding the use of bromelain gel for periodontal disease. Hence, this study has been undertaken for comparative ‘Evaluation of 2% Bromelain gel and 0.2% Chlorhexidine gel as Subgingival Local Drug Delivery following Scaling and Root Planing in Stage II / III and Grade B Periodontitis’ - A Randomized Control Trial.
This prospective randomized trial clinical application was done in the Department of periodontology, Krishnadevaraya College of Dental Sciences and Hospital, Bangalore, Karnataka 562157, India. The present trial was conducted in accordance with the declaration of ethical principles of the World Medical Association Declaration of Helsinki, version VI, 2002. The study was approved by the Institutional Review Board (IRB) and the Ethical Committee (KCDSHEC/IP/2023/V1/P4). The study has been registered at “Clinical Trials.gov” with the identity number NCT06505759.
The study involved the preparation of 2% Bromelain gel and 0.2% Chlorhexidine gel in the Department of Biotechnology, Sir M. Visvesvaraya Institute of Technology, Bengaluru, Karnataka 562157, India.
Preparation
2% Bromelain gel was prepared by adding 250mg of bromelain powder to Carbopol distilled water (200mg+2ml) in a beaker. Methyl paraben was used as a preservative. Triethanolamine was added to modify the pH of the solution. Starch -distilled water (50mg+1ml) was added as a gelatinizing agent. With a dropper, distilled water was added in increments of 1ml in the beaker with constant stirring till a gel-like consistency was formed [14]. Similar protocol was followed for 0.2% Chlorhexidine gel. Prepared gels were subjected to cytotoxicity, and the Minimal Inhibitory Concentration (MIC) of the drug (Bromelain and CHX) was determined.
Prepared gels were stored at 2 °C - 8 °C in a refrigerator until further use. Prepared gels were evaluated at Stroma Biotechnology Pvt.LTD for Minimal Inhibitory Concentration (MIC) and Cytotoxicity. The concentration of Bromelain was set at 2% and Chlorhexidine (CHX) at 0.2%. This was followed by the Clinical application of the gels as Local Drug Delivery (LDD) agents.
Patients with Stage II/III and Grade B periodontitis satisfying the inclusion and exclusion criteria were enrolled for the study. The study subjects underwent the Oral hygiene phase (Scaling and Root Planning (SRP)) and placement of 2% bromelain gel and 0.2% chlorhexidine gel as Local Drug Delivery (LDD) agents.
Inclusion criteria
Patient with Stage II /III and Grade B Periodontitis in the age group of 30-50 years. Patient with ≥20 teeth. Patient has radiographic evidence of horizontal bone loss in at least two quadrants. Localized periodontitis patients with a probing pocket depth (PPD) of ≥5 mm and cooperative, able to attend regular follow-ups were included.
Exclusion criteria
Patients who have received any surgical or nonsurgical therapy. Pregnant or lactating females. Use of systemic antibiotics in the past 6 months. Patients who are not willing to participate in the study.
Clinical Parameters recorded were Plaque index (PI) [15]. Gingival index (GI) [16]. Modified Sulcular Bleeding Index (mSBI) [17]. Probing Pocket Depth (PPD) using UNC-15 probe and Relative attachment level (RAL) using UNC-15 probe. Clinical parameters were recorded following SRP (baseline), at the 4th week, and at the 12th week.
The sample size has been estimated using the GPower software (latest ver. 3.1.9.7; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). The sample size estimation was performed at 5% alpha error (α = 0.05), with an effect size of 52% [Based on the findings from the previous literature, study done by Mega Sibarani, et al. 2020] & the power of study at 80%, revealed that a minimum of 36 samples was necessary for the present study. Anticipating 10% attrition during the follow-up period, the sample size was inflated to 39 samples. So, each group consisted of 12 samples [12 samples x 3 groups = 36 samples].
A total number of 36 probing sites with pocket depth measuring ≥ 5 mm and radiographic evidence of horizontal bone loss were divided into the following groups: Group I (Control) -12 Probing sites with probing depth of ≥ 5 mm and radiographic evidence of horizontal bone loss received SRP alone. Group II-12 Probing sites with probing depth of ≥5mm and radiographic evidence of horizontal bone loss following SRP received 2% Bromelain gel. Group III –12 Probing sites with probing depth of ≥5mm and radiographic evidence of horizontal bone loss following SRP received 0.2% Chlorhexidine gel.
Clinical procedure
Sites were randomly allocated to each group. Computer computer-generated allocation method was followed. Clinical parameters were recorded before SRP and were considered as baseline values. All subjects underwent Scaling and Root Planing by using universal and area-specific curettes and Ultrasonic scalers (PS, miniPiezon, EMS Piezon Systems, Nyon, Switzerland).
12 probing sites received post-operative instructions alone during the maintenance period and were considered as control group. Following SRP, 12 probing sites received 2% bromelain gel, remaining 12 probing sites received 0.2% chlorhexidine gel as an adjunct at the selected site. Oral hygiene instructions were reinforced at regular intervals. Further, patients were recalled for the assessment of the clinical parameters at the 4th week and at the 12th week following periodontal intervention.
Local Drug Delivery (LDD) application
The medicament was delivered at a depth of the pocket of ≥ 5 mm, using a syringe with an applicator tip. After the gel placement, the area was covered with a periodontal pack. (Coe-pack)TM Patients were instructed to refrain from eating for an hour following placement of the gel. Patient was instructed to keep the area clean with a damp cloth only at the treated area till the removal of the periodontal dressing on the 10th day. They were again reinforced with oral hygiene instructions.
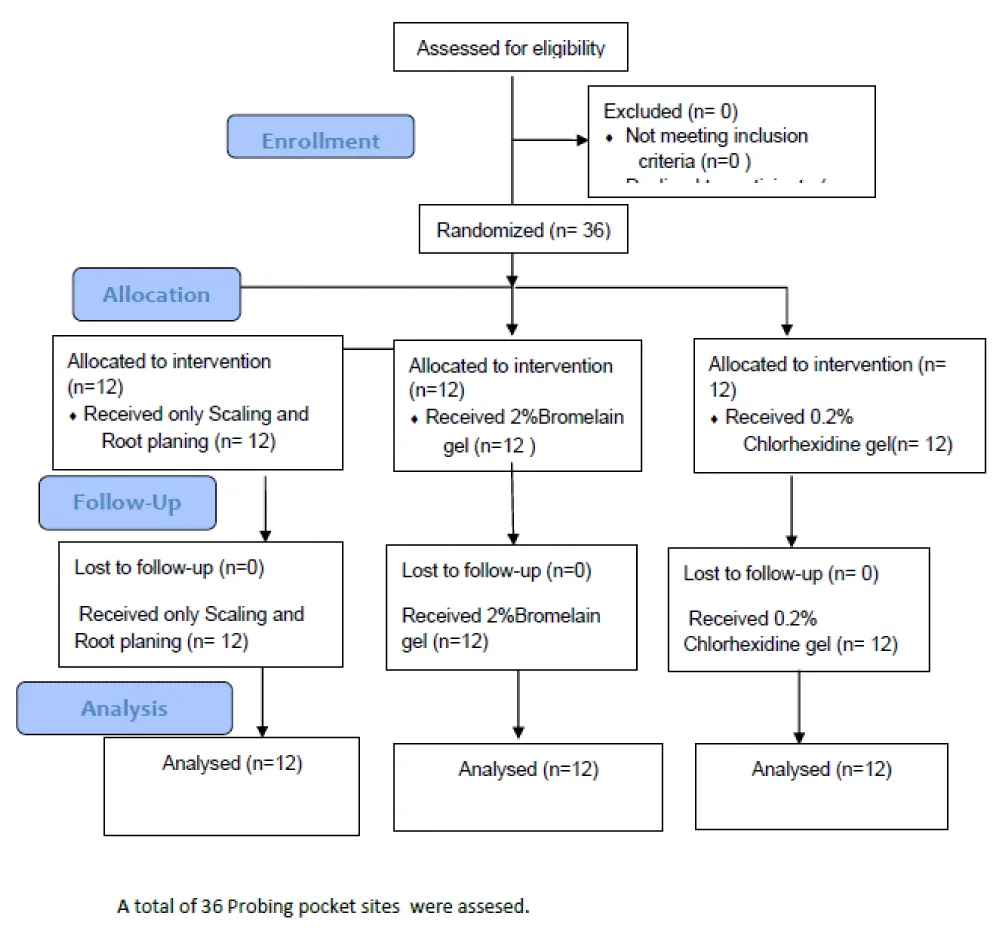
Consort flow chart
Data analysis
Statistical analysis: Statistical Package for Social Sciences [SPSS] for Windows Version 22.0 Released 2013. Armonk, NY: IBM Corp., will be used to perform statistical analyses.
Descriptive statistics: Descriptive statistics were calculated, such as frequency, proportion for categorical data, and mean, standard deviation for continuous data.
Inferential statistics: Sample size was 12 in each group, so a parametric test, the Paired ‘t’ test, was used to compare variables within the group, and an Independent ‘t’ test was used to compare variables between the groups. One-way ANOVA was used to compare more than two variables. Repeated measure of ANOVA followed by a Bonferroni post hoc test was used to compare the means of parameters (PI, GI, mSBI, PPD, and RAL) between different time intervals in each of the groups. p < 0.05 was set for the level of significance. (p - value) Pearson Correlation coefficient (r) was used to compare different variables for the groups at different time intervals.
(Tables 1-5)
| Table 1: Comparison of mean score of different study variables at baseline, week 4 & week 12 of Group I- SRP & Group II- 2% Bromelain Gel. | |||||
| Variables | Group I | Group II | |||
| Mean | Std. Deviation | Mean | Std. Deviation | ||
| PPD | Baseline | 2.9225 | 0.65723 | 2.7058 | 0.42397 |
| Week 4 | 1.2083 | 0.34117 | 0.8208 | 0.25213 | |
| Week 12 | 0.33137 | 0.20 | 0.15204 | 0.22 | |
| RAL | Baseline | 6.8683 | 1.31954 | 7.7333 | 0.89171 |
| Week 4 | 5.0467 | 1.28624 | 5.3000 | 0.67420 | |
| Week 12 | 1.42494 | 2.00 | 0.70754 | 3.20 | |
| PI | Baseline | 0.5833 | 0.24618 | 0.6458 | 0.22508 |
| Week 4 | 0.12500 | 0.226 | 0.0625 | 0.11307 | |
| Week 12 | 0.11307 | 0.00 | 0.11307 | 0.00 | |
| GI | Baseline | 0.5208 | 0.22508 | 0.9500 | 0.24772 |
| Week 4 | 0.0000 | 0.00000 | 0.2917 | 0.17944 | |
| Week 12 | 0.00000 | 0.00 | 0.17944 | 0.00 | |
| mSBI | Baseline | 1.5000 | 0.52223 | 1.4167 | 0.51493 |
| Week 4 | 0.0000 | 0.00000 | 0.4167 | 0.51493 | |
| Week 12 | 0.00000 | 0.00 | 0.51493 | 0.00 | |
| Table 2: Comparison of different variables at different times in Groups II & III. | |||||||||||
| Variables | PPD | RAL | PI | GI | mSBI | ||||||
| Group II | Group III | Group II | Group III | Group II | Group III | Group II | Group III | Group II | Group III | ||
| Baseline | Mean | 2.9225 | 2.7058 | 6.8683 | 7.7333 | 0.5833 | 0.6458 | 0.5208 | 0.95 | 1.5 | 1.4167 |
| SD | 0.65 | 0.424 | 1.3195 | 0.8917 | 0.2462 | 0.2251 | 0.2251 | 0.2477 | 0.5222 | 0.5149 | |
| P Value | 0.35 | 0.04 | 0.52 | <0.0001 | 0.68 | ||||||
| week 4 | Mean | 1.2083 | 0.8208 | 5.0467 | 5.3 | 0.125 | 0.0625 | 0 | 0.2917 | 0 | 0.4167 |
| SD | 0.3412 | 0.2521 | 1.2862 | 0.6742 | 0.2261 | 0.1131 | 0 | 0.1794 | 0 | 0.5149 | |
| P Value | 0.004 | 0.55 | 0.41 | <0.0001 | 0.01 | ||||||
| 12 Weeks | Mean | 0.7833 | 0.4033 | 3.9633 | 4.2333 | 0.0625 | 0.0625 | 0 | 0.2917 | 0.4167 | |
| SD | 0.3314 | 0.152 | 1.4249 | 0.7075 | 0.1131 | 0.1131 | 0 | 0.1794 | 0.5149 | ||
| P Value | 0.02 | 0.56 | 1 | <0.0001 | 0.01 | ||||||
| Table 3: Comparison of mean score of different study variables at baseline between Group II & Group III. | ||||
| Variables | Mean | SD | p value | |
| PPD | Group II | 2.9225 | 0.65723 | 0.35 |
| Group III | 2.7058 | 0.42397 | ||
| RAL | Group II | 6.8683 | 1.31954 | 0.04 |
| Group III | 7.7333 | 0.89171 | ||
| PI | Group II | 0.5833 | 0.24618 | 0.52 |
| Group III | 0.6458 | 0.22508 | ||
| GI | Group II | 0.5208 | 0.22508 | <0.0001 |
| Group III | 0.9500 | 0.24772 | ||
| MsBI | Group II | 1.5000 | 0.52223 | 0.68 |
| Group III | 1.4167 | 0.51493 | ||
| Independent t-test, p - value <0.05 is statistically significant. | ||||
| Table 4: Comparison of mean score of different study variables at 4 weeks between Group II & Group III. | ||||
| Variables | Mean | SD | p value | |
| PPD | Group II | 1.2083 | 0.34117 | 0.004 |
| Group III | 0.8208 | 0.25213 | ||
| RAL | Group II | 5.0467 | 1.28624 | 0.55 |
| Group III | 5.3000 | 0.67420 | ||
| PI | Group II | 0.1250 | 0.22613 | 0.41 |
| Group III | 0.0625 | 0.11307 | ||
| GI | Group II | 0.0000 | 0.00000 | <0.0001 |
| Group III | 0.2917 | 0.17944 | ||
| MsBI | Group II | 0.0000 | 0.00000 | 0.01 |
| Group III | 0.4167 | 0.51493 | ||
| Independent t-test, p - value <0.05 is statistically significant. | ||||
| Table 5: Comparison of mean score of different study variables at 12 weeks between Group II & Group III. | ||||
| Variables | Mean | SD | p value | |
| PPD | Group II | 0.7833 | 0.33137 | 0.02 |
| Group III | 0.4033 | 0.15204 | ||
| RAL | Group II | 3.9633 | 1.42494 | 0.56 |
| Group III | 4.2333 | 0.70754 | ||
| PI | Group II | 0.0625 | 0.11307 | 1.00 |
| Group III | 0.0625 | 0.11307 | ||
| GI | Group II | 0.0000 | 0.00000 | <0.0001 |
| Group III | 0.2917 | 0.17944 | ||
| MsBI | Group II | 0.0000 | 0.00000 | 0.01 |
| Group III | 0.4167 | 0.51493 | ||
| Independent t-test, p - value <0.05 is statistically significant. | ||||
Both groups, Group II and Group III, showed significant reductions in Periodontal Pocket Depth (PPD) from baseline to 12 weeks, indicating the effectiveness of therapy. Group II (2% Bromelain gel) consistently showed greater and faster reduction compared to Group I (SRP alone) and Group III, with statistically highly significant differences at 4 weeks (p = 0.004) and 12 weeks (p = 0.02). This suggests that Bromelain gel enhances healing and pocket reduction when used as an adjunct. Improvement in Relative Attachment Level (RAL) was observed in both groups, though the intergroup difference was not statistically significant (p > 0.05). This indicates both interventions promoted attachment gain, but Bromelain did not show a clear superiority in this parameter. Both groups demonstrated a marked reduction in Plaque Index (PI) over 12 weeks. No significant differences were observed between the groups (p = 1.0 at 12 weeks), showing that plaque control was comparable irrespective of the intervention. Group II exhibited a significantly greater reduction in Gingival Index (GI) scores compared to Group I, particularly at 4 weeks (p < 0.0001). By 12 weeks, Group II maintained significantly better gingival health (p < 0.0001), highlighting the anti-inflammatory effect of Bromelain gel. Gingival bleeding scores significantly reduced in both groups, but Group II showed a greater improvement at both 4 weeks (p = 0.01) and 12 weeks (p = 0.01). This suggests Bromelain’s anti-inflammatory and anti-edematous properties effectively reduce gingival bleeding. Negative correlations were found between clinical parameters (PI, GI, mSBI) and periodontal indices (PPD, RAL), indicating that reductions in plaque and gingival inflammation were associated with improvements in periodontal status. The correlations strengthen the evidence that Bromelain contributes to better control of gingival inflammation and pocket healing.
Adjunctive use of 2% Bromelain gel with scaling and root planing demonstrated superior clinical outcomes compared to SRP alone, particularly in reducing PPD, GI, and mSBI, while maintaining comparable plaque control. 2% Bromelain gel demonstrated early improvement at 4weeks than the CHX group. The findings suggest Bromelain gel as a promising local drug delivery agent with potent anti-inflammatory benefits in the management of periodontitis.
The present study involved the preparation and clinical application of Bromelain gel as a Local drug delivery (LDD) agent in patients having ≥ 5mm probing pocket depth presenting Stage II/III and Grade B periodontitis. Preparation of Bromelain and Chlorhexidine gel (CHX) was conducted in the Department of Biotechnology, Sir M. Visvesvaraya Institute of Technology, Bengaluru. Prepared gel was subjected to determine MIC and Cytotoxicity at Stroma biotechnology PVT LTD. The concentration of Bromelain was at 2% and CHX at 0.2%. Storage of the prepared gel was in refrigeration at 2 °C - 8 °C until use in the Department of Periodontology, Krishnadevaraya College of Dental Sciences and Hospital, Bangalore. Both preparation and clinical application had clearance from the IRB and the Ethical committee with registration in Clinical trials NCT06505759.
All screened patients underwent Scaling and Root planning (SRP) under Phase I therapy following enrolment. 36 Pocket probing sites were considered for statistical analysis. 12 probing sites were followed up on following the SRP alone.
The local application of antimicrobial agents into periodontal pockets, when used along with Scaling and Root planing (SRP), appears to offer additional advantages in reducing Probing depth (PD) and improving Clinical attachment levels (CAL) compared to SRP alone [18]. While several antimicrobial agents for local delivery are commercially available, the ongoing demand for safe, effective, and cost-efficient options has encouraged the exploration of natural extracts. Herbal products and their derivatives, such as pineapple, guava, pomegranate, neem, propolis, Tulsi, green tea, cranberry, and grapefruit, are used in the form of mouthwashes and gels, have demonstrated notable advantages over chemical alternatives in the management of periodontal diseases [19,20].
Pineapple is one of the natural herbal products in which bioactive molecule Bromelain, a proteolytic enzyme which is derived from Hawaiian pineapple stems. Bromelain is classified as a Generally Recognized as Safe (GRAS) substance by the U.S. Food and Drug Administration (FDA). It is commonly available as a dietary supplement and is utilized in pharmaceuticals, cosmetics, and food processing [21]. Bromelain has demonstrated a range of fibrinolytic, antioedematous, antithrombotic, and anti-inflammatory effects in both laboratory and clinical settings. Since its chemical properties were first identified, it has been utilized as a Phytotherapeutic agent. Numerous clinical studies have been conducted to highlight its broad therapeutic potential, with findings suggesting that bromelain may aid in managing various conditions, including cardiovascular disorders, osteoarthritis, diarrhoea, and cancer. Additionally, it has been commonly applied in burn debridement, blood coagulation processes, and immune response modulation [22]. Bromelain also shows antibacterial effect on periodontopathogens [14]. Focussing on these properties, we have prepared 2% Bromelain gel.
Chlorhexidine is a cationic bisbiguanide known for its broad-spectrum antimicrobial properties, low cellular toxicity, and strong binding affinity to skin and mucous membranes [23]. When used over a period of 7 to 10 days in a sustained-release formulation, chlorhexidine has proven effective against subgingival plaque bacteria. Clinically, its benefits include reduced bleeding on probing, decreased pocket depths, and improvements in clinical attachment levels. The antimicrobial action has been observed to last for 6 to 9 months after treatment [24]. In 2021, Hasan F, et al. [25] conducted a randomized clinical trial utilizing 1% chlorhexidine gel as a local drug delivery (LDD) system for treating periodontal disease. Their study demonstrated favourable outcomes, showing reductions in clinical indicators and improvements in both gingivitis and periodontitis. In our study, we have used a prepared 0.2% Chlorhexidine gel as a standard to compare with 2% Bromelain gel as an LDD agent. This formulation enabled the drug to remain in the periodontal pocket for an extended duration.
In the present study, we used 2% Bromelain and 0.2% Chlorhexidine in the form of gel as an adjunct to SRP. Bromelain has been used for oral intake to assess the intestinal absorption of proteins (undegraded) by assessing the presence of bromelain in plasma [26]. In children with acute sinusitis, Bromelain (Bromelain-POS) has been used in a therapeutic regimen with standard regime and has shown encouraging results when compared to monotherapy (standard regime) [27] The anti-inflammatory effect of Bromelain (40mg) has shown advantage following third molar extraction demonstrating its effectiveness in treating post operative oedema and pain when compared to ketoprofen group through oral administration [28]. In our study, we have used 2% Bromelain in gel form for application as LDD in probing pocket depth of ≥5mm and comparing with 0.2% Chlorhexidine (CHX) gel.
In the present study, there was a reduction in Plaque index (PI) in both the Bromelain group and the Chlorhexidine group, with no statistically significant difference between the two groups. Patel C [29], in their 1-month study, to assess the effectiveness of Bromelain mouthrinse on gingival inflammation and bacterial plaque have showed a significant reduction in PI scores and GI scores (P-002 and 0.01) respectively against placebo mouth rinse [29]. Unlike our study using Bromelain gel against CHX gel adjunct to and with SRP alone for 3 months (12 weeks). Both the Bromelain group and the CHX group demonstrated a highly significant reduction in PI and GI score at 1month and 3months.
Reddy VK [30] evaluated the effect of Bromelain gel for caries dentin excavation against papain gel and concluded that the chemico-mechanical property of Bromelain gel was more effective than papain. The proteolytic property of Bromelain has been taken advantage of in their study. Babazade H [31] evaluated the effect of Bromelain (Anaheal 500 mg) capsule following periodontal surgery for 1month. They have assessed PI, GI, bleeding on probing (BOP), and PPD (≥ 5 mm) against placebo at baseline and one week. Reduction in PI and BOP scores was significantly lower with the Anaheal group compared to placebo. In their study, there was no significant difference in GI score (p-0.120); mean PPD was higher in the Anaheal group [31]. In our study, there was a significant reduction of PI, GI, mSBI, and PPD in the Bromelain group and the CHX group at 1month and 3months. Gingival index (GI) and Modified sulcular bleeding index (mSBI) of Bromelain group showed significant reduction when compared with Chlorhexidine group, which is highly significant at 1month and at 3 months, demonstrating the anti-inflammatory property of Bromelain. Systematic review and meta-analysis by De Souza, et al. [32] have proved the effect of bromelain in reducing pain and odema.
Chisci G [33] evaluated the therapeutic efficacy of oral bromelain (Ananase Angelini 40 mg) in alveolar ridge preservation after tooth extraction. In their study, pain and postoperative swelling were evaluated for 2 weeks at 2,7, and 14th days. Bromelain reported better results than the control (paracetamol). Generally, Cortisol efficacy in pain and swelling would be offset by the effect of the reduction of tissue mineralization, leading to bone resorption [34]. Benefits of Bromelain equal non-steroidal anti-inflammatory drugs (NSAIDS), wound healing, trauma, surgery, and deep burns [35]. The regenerative potential of Bromelain has been highlighted in their study. Similarly, in our study, we have evaluated 2% Bromelain gel as an adjunct to SRP with encouraging outcomes (PI, GI, mSBI, PPD, and RAL).
Taneja S, et al. [36] evaluated in their in vitro study on the effect of using natural proteolytic enzymes on surface structure and microhardness of cementum of avulsed tooth. 5.2% Sodium Hypochlorite (NaOCL), 10% bromelain, 10% papain, and 10% panzyme were used on the tooth root surface topography and microhardness. Their study concluded that 10% bromelain for 10 min was effective in the removal of necrotic PDL fibres, preserving the cemental integrity. In our study, we have used 2% bromelain gel for application in ≥5mm periodontal pockets as an LDD agent has shown promising results as an adjunct to SRP Intergroup comparison with CHX has shown reduction in Probing pocket depth (PPD) and Relative attachment level (RAL) in both Bromelain gel and Chlorhexidine gel at 1month and 3 months which is highly statistically significant.
Amr K. Ahmed, et al. [37] in their study on Quadruple therapy (capsules) consisting of Zinc, Quercetin, Bromelain, and Vitamin C showed promising results in improving clinical outcome among COVID-19 patients. In our study, 2% bromelain gel and 0.2% CHX gel application has demonstrated reduction in clinical parameters (PI, GI, mSBI, PPD, and RAL) at 1month and 3 months. Both studies show positive outcomes, directing a promising future for Bromelain usage (regenerative potential). Soulissa AG, et al. [38] evaluated the antibacterial and antibiofilm efficacy of Pineapple hump on Porphyromonas gingivalis. They concluded that the extract of pineapple hump inhibited bacterial growth and eradicated the adhesion of P. gingivalis biofilms. The antimicrobial effect of bromelain enzyme has been confirmed through this study. Our study has confirmed the anti-inflammatory property (GI, mSBI) of Bromelain gel along with positive clinical outcome (PPD and RAL). Hugar SS, et al. [39] Interventional study on application of 0.2% CHX gel and Curcumin gel as adjunct to SRP showed significant reduction in GI score for both groups. The curcumin group showed better results (PI, SBI, PPD) than the CHX group. In our study, we have compared SRP adjunct 2% Bromelain and 0.2% CHX with SRP alone. Siddharth M [40] comparatively evaluated 2% Curcumin and 0.2% CHX gel as an adjunct to SRP in Chronic periodontitis patients. They have demonstrated curcumin as a better herbal agent than CHX and suggest curcumin gel adjunct to SRP in moderate periodontitis and periodontal maintenance as LDD. Our study also shows similar results with 2% Bromelain gel and 0.2% CHX gel adjunct to SRP than SRP alone, as compared to both the above studies.
The present study met all the objectives of the study. Our study was not without limitations, a smaller sample size (36 probing sites) and a shorter duration of follow-up after LDD (3 months). Microbial evaluation was not done. Further clinical trials with a larger sample size and longer follow-up with microbial analysis will definitely benefit the periodontal patients at large. Various findings from traditional and clinical reports indicate that bromelain and its combination may be an effective agent to improve periodontal health. Further, its potential in tissue repair and regeneration needs to be explored. Studies should incorporate a larger sample size with probing pocket depth (6mm), ensure more robust and generalizable results with a comprehensive microbiological analysis validate its antimicrobial efficacy.
- Caton JG, Armitage G, Berglundh T, Chapple IL, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions: introduction and key changes from the 1999 classification. Journal of Periodontology. 2018;89(S1):S1–S8. Available from: https://doi.org/10.1111/jcpe.12935
- Highfield J. Diagnosis and classification of periodontal disease. Australian Dental Journal. 2009;54(S1):S11–S26. Available from: https://doi.org/10.1111/j.1834-7819.2009.01140.x
- Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontology 2000. 1994;5(1):78–111. Available from: https://doi.org/10.1111/j.1600-0757.1994.tb00020.x
- Baehni PC. Supportive care of the periodontal patient. Current Opinion in Periodontology. 1997;4:151–157.
- Pasupuleti MK, Nagate RR, Alqahtani SM, Penmetsa GS, Gottumukkala SN, Ramesh KS. Role of medicinal herbs in periodontal therapy: a systematic review. Journal of the International Society of Preventive and Community Dentistry. 2023;13(1):9–16. Available from: https://doi.org/10.4103/jispcd.jispcd_210_22
- Kolliyavar B, Shettar L, Thakur S. Chlorhexidine: the gold standard mouthwash. Journal of Pharmaceutical and Biomedical Sciences. 2016;6(2). Available from: https://www.semanticscholar.org/paper/Chlorhexidine%3A-The-Gold-Standard-Mouth-Wash-Kolliyavar-Shettar/19852841f265810616fd666b34ae1b74594e00a1
- Mensi M, Palazzolo A, Garzetti G, Lops D, Calza S, Rota M. Xanthan-based chlorhexidine gel effects in non-surgical periodontal therapy: a meta-analysis. Oral Diseases. 2024;30(6):3813–3827. Available from: https://doi.org/10.1111/odi.14956
- Annisa ZU, Sulijaya B, Tadjoedin ES, Hutomo DI, Masulili SL. Effectiveness of chlorhexidine gels and chips in periodontitis patients after scaling and root planing: a systematic review and meta-analysis. BMC Oral Health. 2023;23(1):819. Available from: https://doi.org/10.1186/s12903-023-03241-2
- Budală DG, Luchian I, Tatarciuc M, Butnaru O, Armencia AO, Virvescu DI, et al. Are local drug delivery systems a challenge in clinical periodontology? Journal of Clinical Medicine. 2023;12(12):4137. Available from: https://doi.org/10.3390/jcm12124137
- Rangrej U, Dave D, Rai J, Vaghani K. Evidence-based review on herbal local drug delivery. 2017. Available from: https://www.semanticscholar.org/paper/Evidence-Based-Review-on-Herbal-Local-Drug-Delivery-Rangrej-Dave/53d3884429c55b9f0aff84412490308e23e60bf4
- Taussig SJ, Batkin S. Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application: an update. Journal of Ethnopharmacology. 1988;22(2):191–203. Available from: https://doi.org/10.1016/0378-8741(88)90127-4
- Braun JM, Schneider B, Beuth HJ. Therapeutic use, efficiency, and safety of the proteolytic pineapple enzyme Bromelain-POS in children with acute sinusitis in Germany. In Vivo. 2005;19(2):417–421. Available from: https://pubmed.ncbi.nlm.nih.gov/15796206/
- Rathnavelu V, Alitheen NB, Sohila S, Kanagesan S, Ramesh R. Potential role of bromelain in clinical and therapeutic applications. Biomedical Reports. 2016;5(3):283–288. Available from: https://doi.org/10.3892/br.2016.720
- Praveen NC, Rajesh A, Madan M, Chaurasia VR, Hiremath NV, Sharma AM. In vitro evaluation of antibacterial efficacy of pineapple extract (bromelain) on periodontal pathogens. Journal of International Oral Health. 2014;6(5):96–100. Available from: https://pubmed.ncbi.nlm.nih.gov/25395802/
- Silness J, Löe H. Periodontal disease in pregnancy II: correlation between oral hygiene and periodontal condition. Acta Odontologica Scandinavica. 1964;22(1):121–135. Available from: https://doi.org/10.3109/00016356408993968
- Löe H, Silness J. Periodontal disease in pregnancy I: prevalence and severity. Acta Odontologica Scandinavica. 1963;21(6):533–551. Available from: https://doi.org/10.3109/00016356309011240
- Mühlemann HR, Son S. Gingival sulcus bleeding: a leading symptom in initial gingivitis. Helvetica Odontologica Acta. 1971;15(2):107–113. Available from: https://pubmed.ncbi.nlm.nih.gov/5315729/
- Hanes PJ, Purvis JP. Local anti-infective therapy: pharmacological agents. A systematic review. Annals of Periodontology. 2003;8(1):79–98. Available from: https://doi.org/10.1902/annals.2003.8.1.79
- Kukreja BJ, Dodwad V. Herbal mouthwashes: gift of nature. International Journal of Pharma and Bio Sciences. 2012;3(2):46–52. Available from: https://www.ijpbs.net/abstract.php?article=MTI3NQ==
- Reddy PD, Satyanarayana T, Swarna LD, Purushothaman M. Local drug delivery of herbs for treatment of periodontitis. Journal of Innovative Trends in Pharmaceutical Sciences. 2010;1:245–251. Available from: https://www.scribd.com/document/507606642/Local-Drug-Delivery-of-Herbs-for-Treatment-of-Periodontitis
- Kansakar U, Trimarco V, Manzi MV, Cervi E, Mone P, Santulli G. Exploring the therapeutic potential of bromelain: applications, benefits, and mechanisms. Nutrients. 2024;16(13):2060. Available from: https://doi.org/10.3390/nu16132060
- Pavan R, Jain S, Shraddha, Kumar A. Properties and therapeutic application of bromelain: a review. Biotechnology Research International. 2012;2012:976203. Available from: https://doi.org/10.1155/2012/976203
- Jones CG. Chlorhexidine: is it still the gold standard? Periodontology 2000. 1997;15:55–62. Available from: https://doi.org/10.1111/j.1600-0757.1997.tb00105.x
- Narayanan R, Prabhuji ML, Bhavikatti SK, Saquib SA, Paramashivaiah R, Karobari MI, et al. Clinical evaluation of local drug delivery of chlorhexidine and ornidazole in the treatment of gingivitis as an adjunct to scaling and root planing. Indian Journal of Forensic Medicine and Toxicology. 2020;14(4):4346–4353. Available from: https://medicopublication.com/index.php/ijfmt/article/view/12322
- Hasan F, Ikram R, Adel A, Abbas A, Ain Bukhari QU, Asadullah K. Treatment of periodontal diseases by the local drug delivery system using 1% chlorhexidine gel: a randomized clinical trial. Pakistan Journal of Pharmaceutical Sciences. 2021;34(1):41–45. Available from: https://pubmed.ncbi.nlm.nih.gov/34248001/
- Castell JV, Friedrich GE, Kuhn CS, Poppe GE. Intestinal absorption of undegraded proteins in men: presence of bromelain in plasma after oral intake. American Journal of Physiology–Gastrointestinal and Liver Physiology. 1997;273(1):G139–G146. Available from: https://doi.org/10.1152/ajpgi.1997.273.1.g139
- Braun JM, Schneider B, Beuth HJ. Therapeutic use, efficiency, and safety of the proteolytic pineapple enzyme Bromelain-POS in children with acute sinusitis in Germany. In Vivo. 2005;19(2):417–421. Available from: https://pubmed.ncbi.nlm.nih.gov/15796206/
- Inchingolo F, Tatullo M, Marrelli M, Inchingolo AM, Picciariello V, Inchingolo AD, et al. Clinical trial with bromelain in third molar exodontia. European Review for Medical and Pharmacological Sciences. 2010;14(9):771–774. Available from: https://pubmed.ncbi.nlm.nih.gov/21061836/
- Patel C, Kumaresan S. Effectiveness of bromelain mouthrinse in gingival inflammation and bacterial plaque among adolescents: a randomized clinical trial. 2022. Available from: https://static1.squarespace.com/static/6219994322f73643a739a431/t/6365eaed3d9e67600148cb61/1667623663526/Int%2BJ%2BComm%2BDent%2B2022%2B10%282%2964-%2B69.pdf
- Reddy VK, Nagar P, Reddy S, Ragulakollu R, Tirupathi SP, Ravi R, et al. Bromelain vs papain gel for caries removal in primary teeth. The Journal of Contemporary Dental Practice. 2019;20(11):1343–1349. Available from: https://pubmed.ncbi.nlm.nih.gov/31892689/
- Babazade H, Mirzaagha A, Konarizadeh S. The effect of bromelain in periodontal surgery: a double-blind randomized placebo-controlled trial. BMC Oral Health. 2023;23(1):286. Available from: https://doi.org/10.1186/s12903-023-02971-7
- De Souza GM, Fernandes IA, dos Santos CR, Falci SG. Is bromelain effective in controlling the inflammatory parameters of pain, edema, and trismus after lower third molar surgery? A systematic review and meta-analysis. Phytotherapy Research. 2019;33(3):473–481. Available from: https://pubmed.ncbi.nlm.nih.gov/30484910/
- Chisci G, Fredianelli L. Therapeutic efficacy of bromelain in alveolar ridge preservation. Antibiotics. 2022;11(11):1542. Available from: https://doi.org/10.3390/antibiotics11111542
- Klongnoi B, Kaewpradub P, Boonsiriseth K, Wongsirichat N. Effect of single dose preoperative intramuscular dexamethasone injection on lower impacted third molar surgery. Int J Oral Maxillofac Surg. 2012;41(3):376–379. Available from: https://doi.org/10.1016/j.ijom.2011.12.014
- Abdul Muhammad Z, Ahmad T. Therapeutic uses of pineapple-extracted bromelain in surgical care review. J Pak Med Assoc. 2017;67(1):121. Available from: https://pubmed.ncbi.nlm.nih.gov/28065968/
- Taneja S, Dudeja C, Bhalla VK, Taneja P. Does periodontal ligament removal using natural proteolytic enzymes alter the surface structure and microhardness of cementum of avulsed tooth? An in vitro analysis. Saudi Endod J. 2023;13(1):57–62. Available from: https://journals.lww.com/senj/fulltext/2023/13010/does_periodontal_ligament_removal_using_natural.8.aspx
- Kamel A, Abdelseed H, Albalawi Y, Aslsalameen E, Almutairi Y, Alkattan A. Evaluation of the effect of zinc, quercetin, bromelain, and vitamin C on COVID-19 patients. MedRxiv. 2020;2020–12. Available from: https://doi.org/10.1101/2020.12.22.20245993
- Soulissa AG, Lombardo B, Widyarman AS. Antibacterial and antibiofilm efficacy of pineapple hump (Ananas comosus) on Porphyromonas gingivalis in vitro. J Dent Indones. 2021;28(3):153–157. Available from: https://scholarhub.ui.ac.id/jdi/vol28/iss3/4/
- Hugar SS, Patil S, Metgud R, Nanjwade B, Hugar SM. Influence of application of chlorhexidine gel and curcumin gel as an adjunct to scaling and root planing: an interventional study. J Nat Sci Biol Med. 2016;7(2):149. Available from: https://doi.org/10.4103/0976-9668.184701
- Siddharth M, Singh P, Gupta R, Sinha A, Shree S, Sharma K. A comparative evaluation of subgingivally delivered 2% curcumin and 0.2% chlorhexidine gel adjunctive to scaling and root planing in chronic periodontitis. J Contemp Dent Pract. 2020;21(5):494–499. Available from: https://pubmed.ncbi.nlm.nih.gov/32690830/